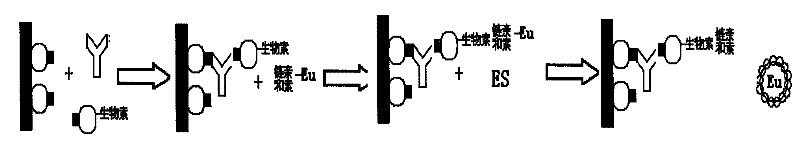Hepatitis c virus antibody time-resolved fluoroimmunoassay and kit
A hepatitis C virus, time-resolved technology, applied to the analysis of materials, measuring devices, instruments, etc., can solve the problems of poor stability, cumbersome operation, and low sensitivity, and achieve small cross-reaction, short detection time, and good specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
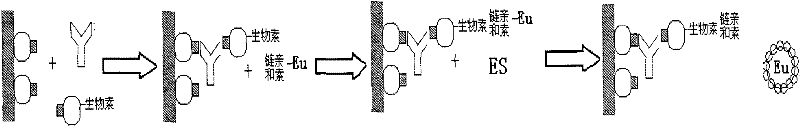
Image
Examples
preparation example Construction
[0025] Next is the preparation of HCV biotin-labeled antigen, including the following steps:
[0026] (1) Add the antigen to be labeled into the dialysis bag, put it into the prepared pH 9.5 carbonate buffer, and dialyze overnight at room temperature (pH 9.5 carbonate buffer);
[0027] (2) The next day, take out the antigen, dilute the dialyzed antigen with pH 9.5 carbonate buffer to 0.2-5 mg / mL, and add biotin while shaking at a mass ratio of 2:1 (biotin: antigen). At room temperature, vibrate slowly on a vibrating plate machine for 1 hour, then dialyze overnight in phosphate buffered saline (pH7.5 phosphate buffer) after removal;
[0028] (5) filter with a filter with an aperture of 0.2um;
[0029] (6) Add 0.3% (W / V) premium pure BSA and store at 2-8°C.
Embodiment
[0030] Example: Hepatitis C virus antibody detection
[0031] 1. Biotin-labeled antigen
[0032] Dialyze the HCV recombinant antigen with carbonate buffer solution overnight, then add biotin to react for 1 hour, take it out and dialyze overnight in phosphate buffer solution with pH 7.5. Aliquot and store at +2~+8℃.
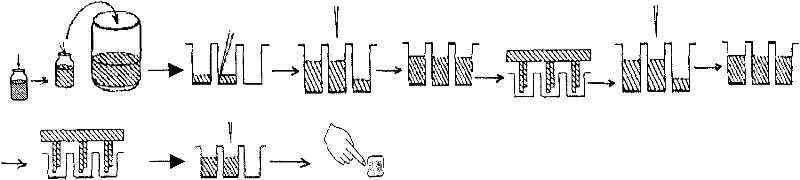
[0033] 2. Operation steps
[0034] 1.) Kit preparation: antigen-coated buffer (carbonate buffer), blocking solution (phosphate buffer and 2% (W / V) BSA), reaction buffer (50mmol / L of pH 7.8 Tris-HCl, containing 0.9% NaCl, 1% BSA, 0.5% casein, 0.08% Tween20 and 0.1% NaN 3 ), working washing solution (phosphate buffer saline), fluorescence enhancing solution (β-diketone body), as streptavidin coated with HCV recombinant antigen, biotin-labeled antigen and lanthanide ion-labeled.
[0035] A: Working washing solution: dilute 40mL phosphate buffer solution (25 times concentrate) with distilled or deionized water to 1,000mL for later use.
[0036] B: Biotin-labeled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com