Hepatitis C virus antibody detection kit, preparation method and detection method
A hepatitis C virus and antibody detection technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of low sensitivity and poor specificity, and achieve the effect of improving detection sensitivity and combining fully
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A hepatitis C virus antibody detection kit, including R1 reagent, R2 reagent, R3 reagent, calibrator and quality control product, wherein,
[0073] The R1 reagent includes a solution of streptavidin magnetic particles, the concentration of the streptavidin magnetic particles is 0.05-1 mg / ml, and the particle diameter of the magnetic particles is 1-4 μm.
[0074] R2 reagents include a mixed reagent of biotinylated hepatitis C virus antigen and hepatitis C virus recombinant antigen, the concentration of biotinylated hepatitis C virus antigen is 0.05-0.2%, and the concentration of hepatitis C virus recombinant antigen is 0.05-0.2% . Biotinylated hepatitis C virus antigen is obtained by tandem fusion expression of HCV Core, NS3, NS4, and NS5 gene fragments, and hepatitis C virus recombinant antigen is obtained by tandem fusion expression of HCV Core, NS3, NS4, and NS5 gene fragments. The hepatitis C virus antigen and the hepatitis C virus recombinant antigen bind to the he...
Embodiment 2
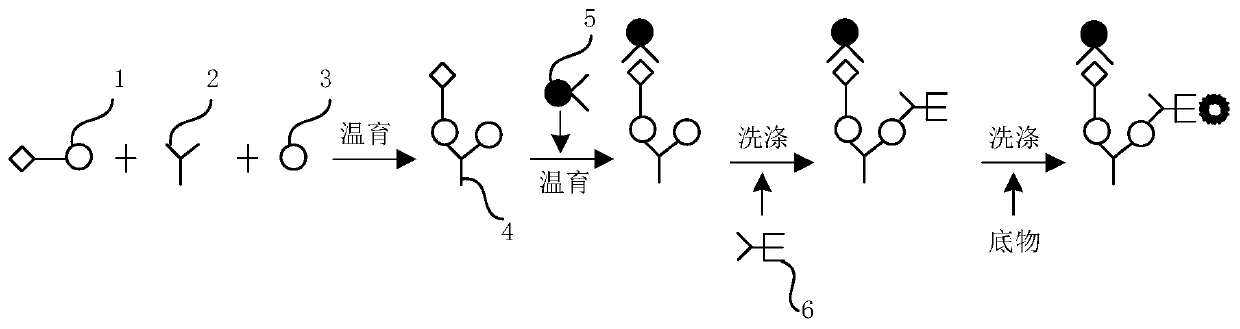
[0101] Detection method of hepatitis C virus antibody detection kit
[0102] Step A: Mix the sample to be tested with R2 reagent, incubate at 36-38°C, biotinylated hepatitis C virus antigen and hepatitis C virus recombinant antigen and hepatitis C virus antibody in the test object respectively to form "antigen- Antibody-antigen "sandwich complexes;
[0103] Step B: Add R1 reagent streptavidin-labeled magnetic particles and incubate at 36-38°C. The above-mentioned sandwich complex is bridged by the affinity between biotin and streptavidin, so that the complex is linked on the solid surface. phase carrier, wash;
[0104] Step C: adding R3 reagent alkaline phosphatase-labeled monoclonal antibody to hepatitis C virus, and washing;
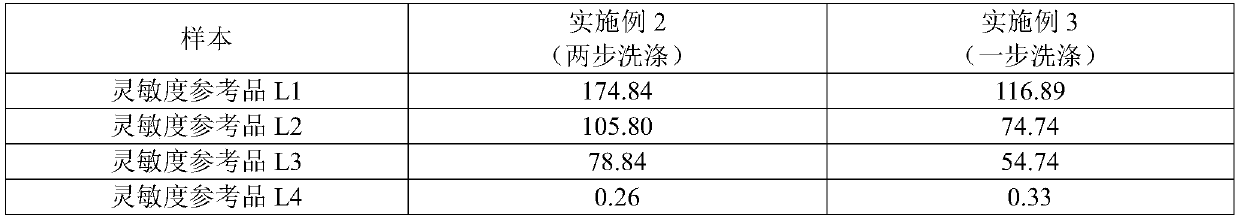
[0105] Step B and step C are carried out sequentially, two-step washing;
[0106] Step D: Add the chemiluminescent substrate AMPPD to make it catalyzed by the enzyme to generate a luminescent signal, which is received by the optical quantum reading ...
Embodiment 3
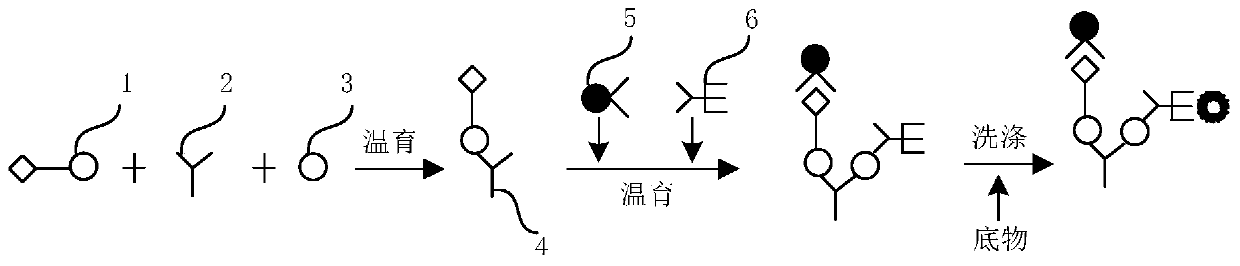
[0108] Detection method of hepatitis C virus antibody detection kit
[0109] Step B and step C are carried out at the same time, adding R1 reagent streptavidin-labeled magnetic particles and R3 reagent alkaline phosphatase-labeled hepatitis C virus monoclonal antibody, incubating at 36-38°C, and washing in one step. All the other steps are the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com