Patents
Literature
36 results about "Hepatitis C Virus Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
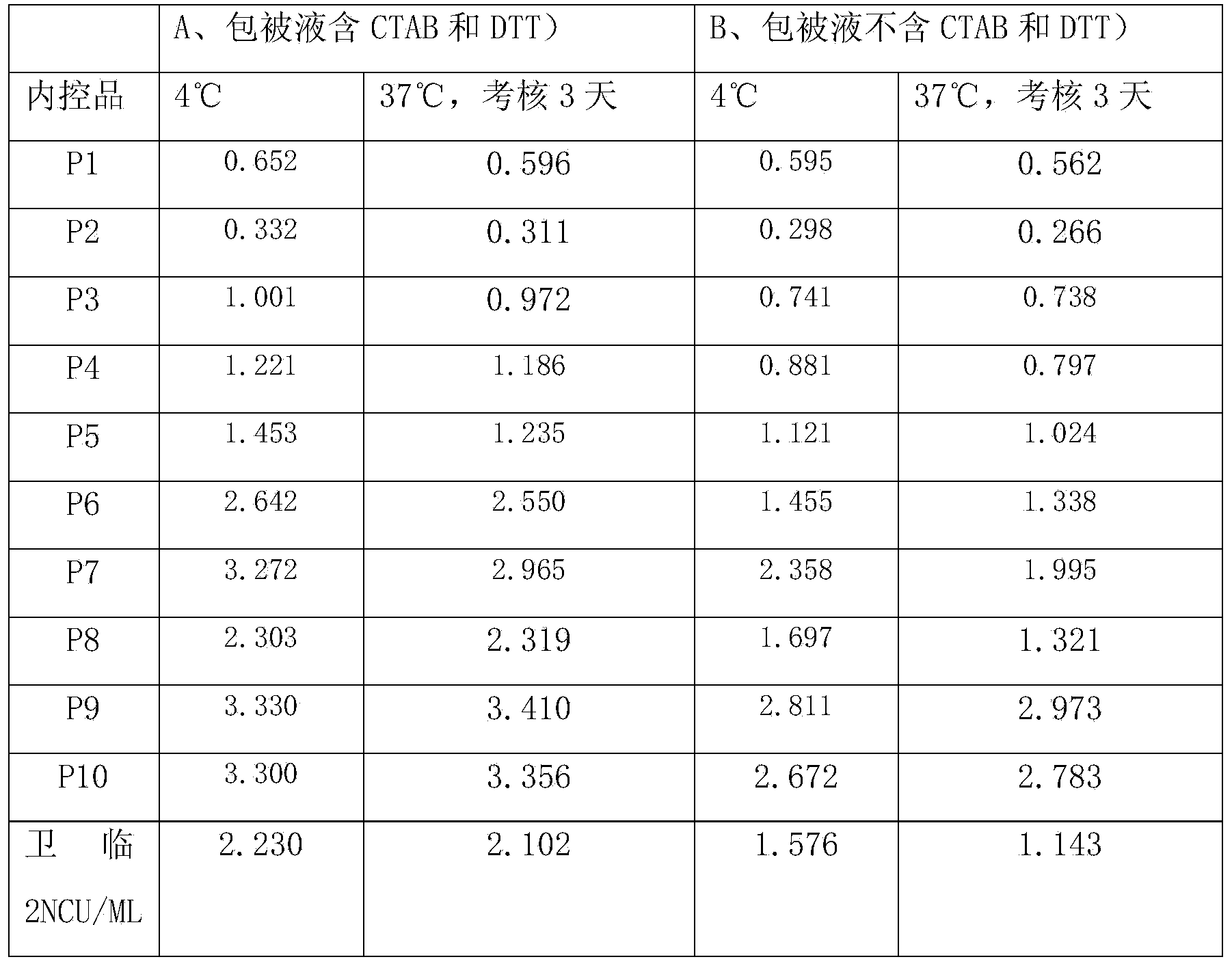
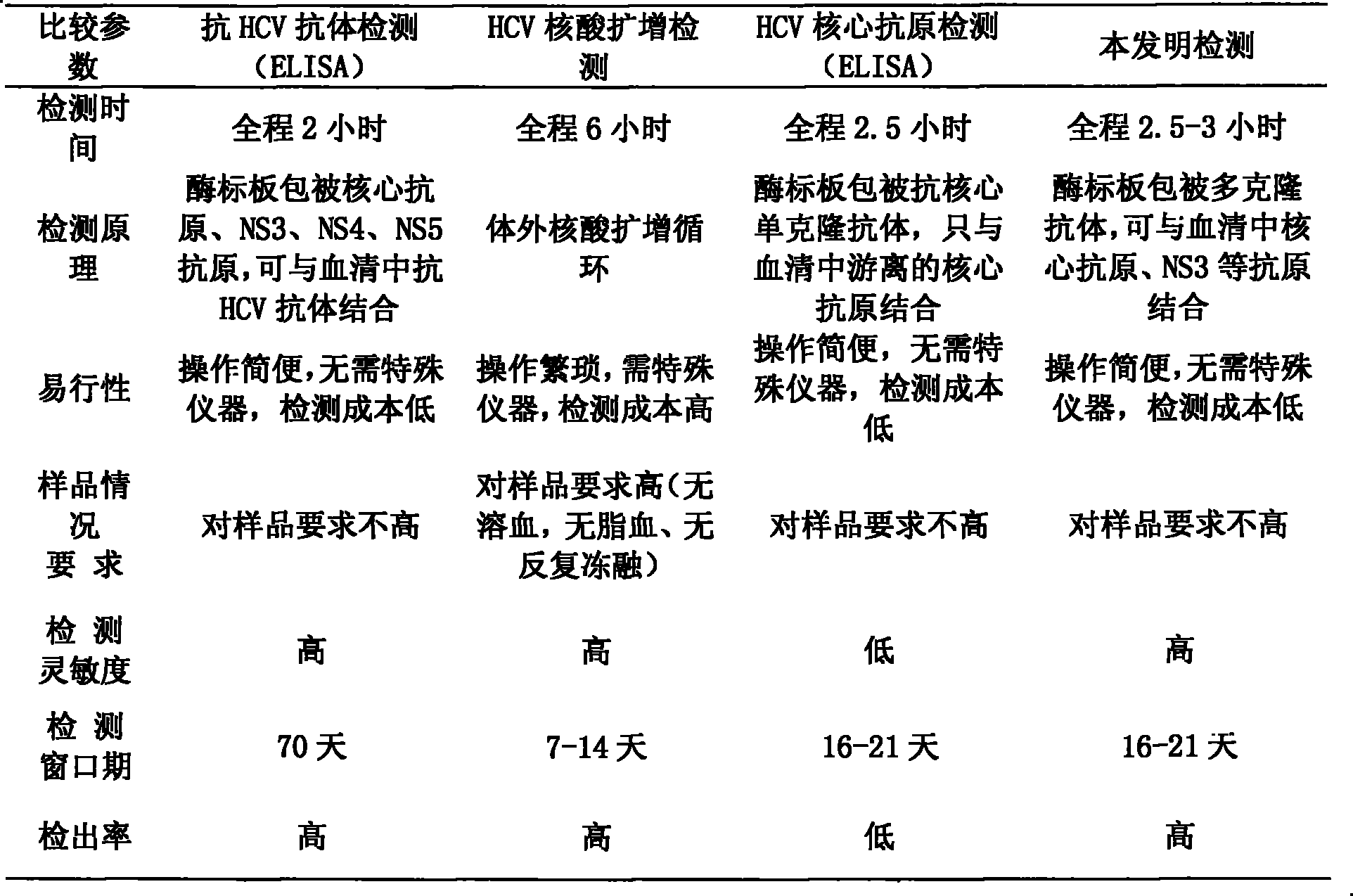
The hepatitis C core antigen is a viral protein. Since the core antigen is part of hepatitis C virus, it can usually be found in the bloodstream two weeks after infection. Since HCV core antigen testing is simpler and less expensive than viral-load testing, some experts suggest using it in resource-limited settings.
Hepatitis C virus antigen-antibody combined detection method
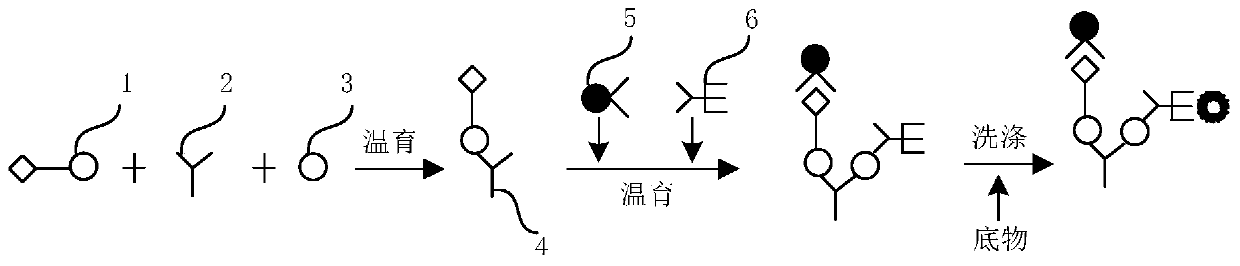
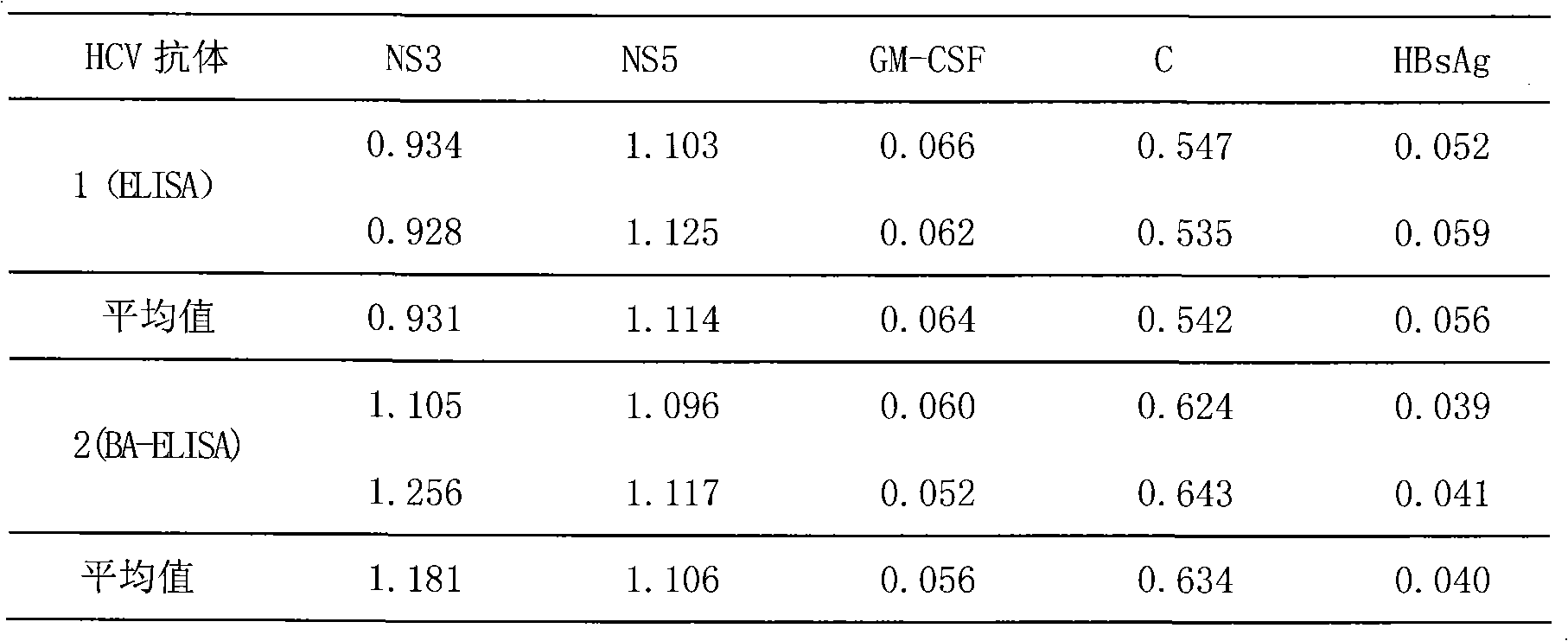
The invention discloses a method for jointly detecting hepatitis C virus (HCV) antigen-antibody. A monoclonal antibody of an anti-HCV core antigen and a chimeric antigen are coated with enzyme-linked plate to be detected simultaneously, so that the method can shorten the 'window period' of HCV virus detection. Moreover, HCV antibodies in serum and the compatibility with the coated monoclonal antibody can be detected as possible so as to avoid the combination of the chimeric antigen and the monoclonal antibody by modifying HCV overall length core antigen, telescoping non-structural areas and realizing the expression of the chimeric antigen, and the positive detection rate is close to the PCR positive detection rate. The HCV detection method also has the advantages of simple operation, low price, so the method is suitable for promotion and application.
Owner:湖南景达生物工程有限公司
Hepatitis C virus antigen-antibody combined detection kit and detection method
ActiveCN103630690AImprove blood consumptionImprove securityBiological material analysisAntigenTherapy Evaluation
The invention discloses a hepatitis C virus (HCV) antigen-antibody combined detection kit. The kit comprises a microwell plate coated with an HCV chimeric antigen and an HCV monoclonal antibody, a sample diluent, an HCV antigen-antibody combined detection enzyme working fluid, an HCV abzyme working fluid, an HCV antigen enzyme working fluid, a substance A fluid, a substance B fluid, a 20-times concentrated washing fluid and a stopping fluid. The invention also discloses preparation and usage of the key components of the kit, such as the microwell plate of the HCV chimeric antigen and the HCV monoclonal antibody, the sample diluent, and the diluent of enzyme working fluid. The kit and detection method, disclosed by the invention, are able to be used for detecting the HCV antigen and antibody at the same time, or individually detecting the situation of the HCV antigen during the early hepatitis c or the acute infection period, or before the antibody is produced, or when an antigen-antibody compound is produced, or individually detecting the situation of the HCV antibody after the antibody is produced; the kit and detection method can be applied to early HCV detection and therapy evaluation, so as to provide important detection evaluation index for clinical guideline.
Owner:山东莱博生物科技有限公司
Method for detecting third type hepatitis virus antibody by using magnetic micro-particle as transporting species
InactiveCN101551394AThe detection process is fastStrong specificityChemiluminescene/bioluminescenceRadioactive contaminationAntigen-Antibody Complex
The present invention discloses a method for detecting third type hepatitis virus antibody by using magnetic micro-particle as transporting species which includes steps as follows: (1) using magnetic micro-particle as transporting species for reacting and separating, coupling third type hepatitis virus antibody on the magnetic micro-particle surface; (2) adding into confining liquid, oscillating 10 min with middle speed under condition of 20-40 deg c, magnetic separating, giving up supernatant; (3) combining the third type hepatitis virus antibody packed on the magnetic micro-particle surface with the specificity antibody on waited blood; (4) separating the magnetic micro-particle with specificity antigen-antibody complex on surface through an externally-applied magnetic field; (5) combining the antibody complex on the magnetic micro-particle surface with enzyme label rat mouse-anti-body IgG; (6) using chemiluminescence detector, irradiancy substrates A liquor and B liquor for detecting sample irradiancy value. The method has characteristics of rapid detecting speed, high specificity, stabilising, no radioactive pollution which can increase sensitivity and stabilizing ability for detecting.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Combination hepatitis c virus antigen and antibody detection method
InactiveUS20080113339A1Microbiological testing/measurementImmunoglobulinsWhole blood productHepatitis C virus core
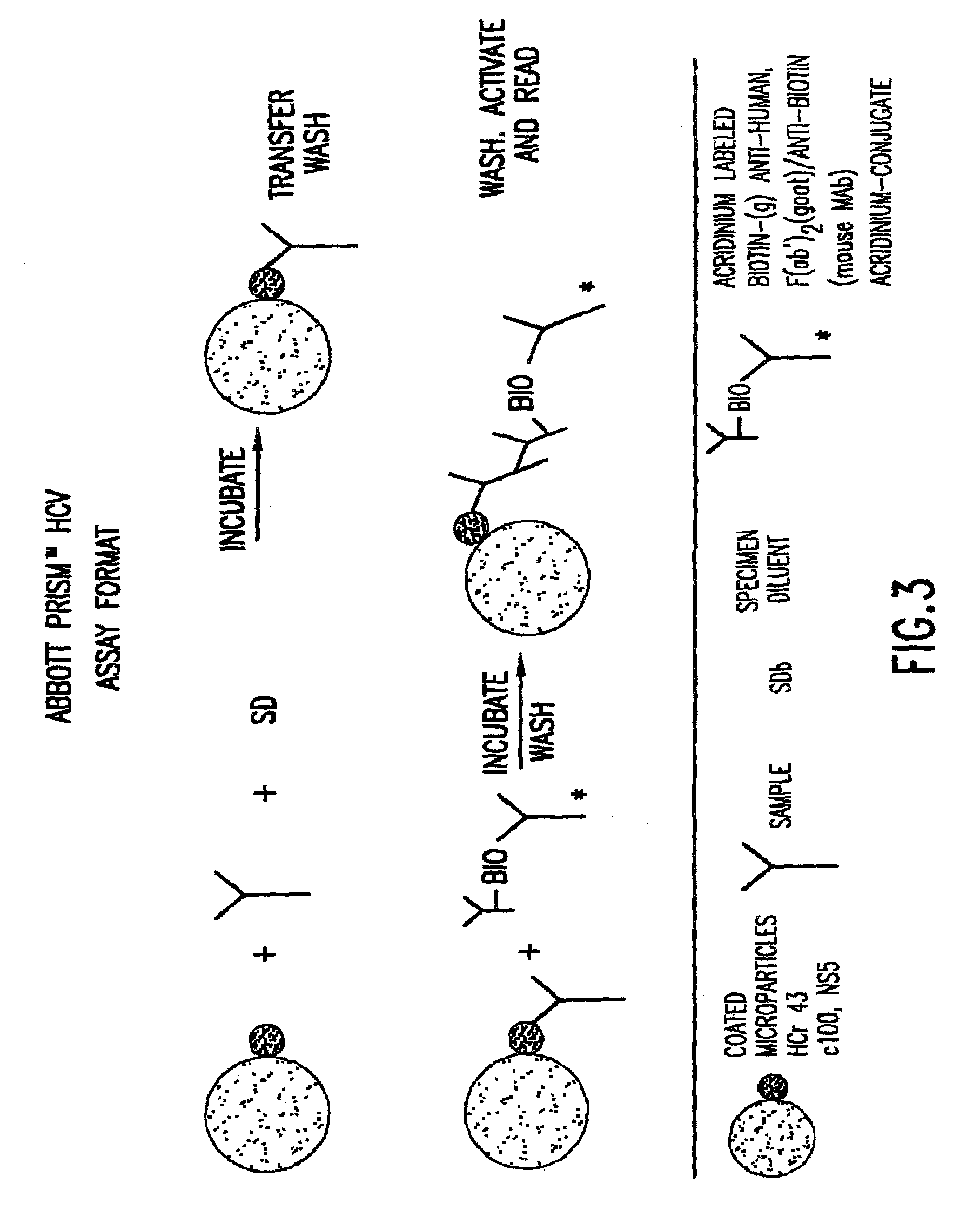
An in vitro method that allows detection of hepatitis C by detecting hepatitis C virus (HCV) core protein and antibodies to HCV core protein (anti-core antibodies) in a single assay is provided. Cross-reactivity is eliminated in the method preferably by utilizing short peptides, each of which has an amino acid sequence that corresponds to an immunodominant region of the native core protein but which does not wholly encompass the epitope bound by the antibodies utilized in the method. The method can be used to detect the presence of HCV in a subject, and / or to determine the suitability of donor blood or blood products for transfusion purposes. Also provided are diagnostic kits for carrying out the method and a process for selecting suitable capture peptides and monoclonal antibodies for use in the combination method.
Owner:ABBOTT LAB INC
Hepatitis c virus antigen-antibody joint detection reagent box and preparation method thereof
ActiveCN104237520AThe "window period" is shortenedHigh detection specificityMaterial analysisAntigenPolyethylene glycol
A hepatitis c virus antigen-antibody joint detection reagent box is characterized by comprising a calibrator(1), a double-marker enzyme conjugate (2), a negative and positive contrast (3), light-emitting liquid (4) and a micropore coated plate (5), wherein the light-emitting liquid contains light-emitting liquid 1 and light-emitting liquid 2, the light-emitting liquid 1 contains luminal 0.7 g / L, cinnamic acid 0.9 g / L, 4-iodophenylboronic acid 0.2 g / L, iodobiphenol 0.25 g / L, dimethylformamide 25 ml / L, polyving akohol 5 g / L, polyvinylpyrrolidone 8 g / L, polyethylene glycol 600 3 g / L, ethylenediamine tetraacetic acid 4 g / L, gentamicin sulfate 1600 thousands / L, urea peroxide 0.4 g / L, and pH 9.0 Tris buffer solution 0.1 mol / L. The light-emitting liquid 2 contains acridinium ester derivative 0.1 mg / ml, polyethylene glycol 600 3 g / L and 0.1 mol / L of pH 9.0 Tris buffer solution containing 0.1% of TWEEN-20. The invention further discloses a preparation method and a using method of the reagent box. The hepatitis c virus antigen-antibody joint detection reagent box has the advantages of being quick in reaction and low in cost.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
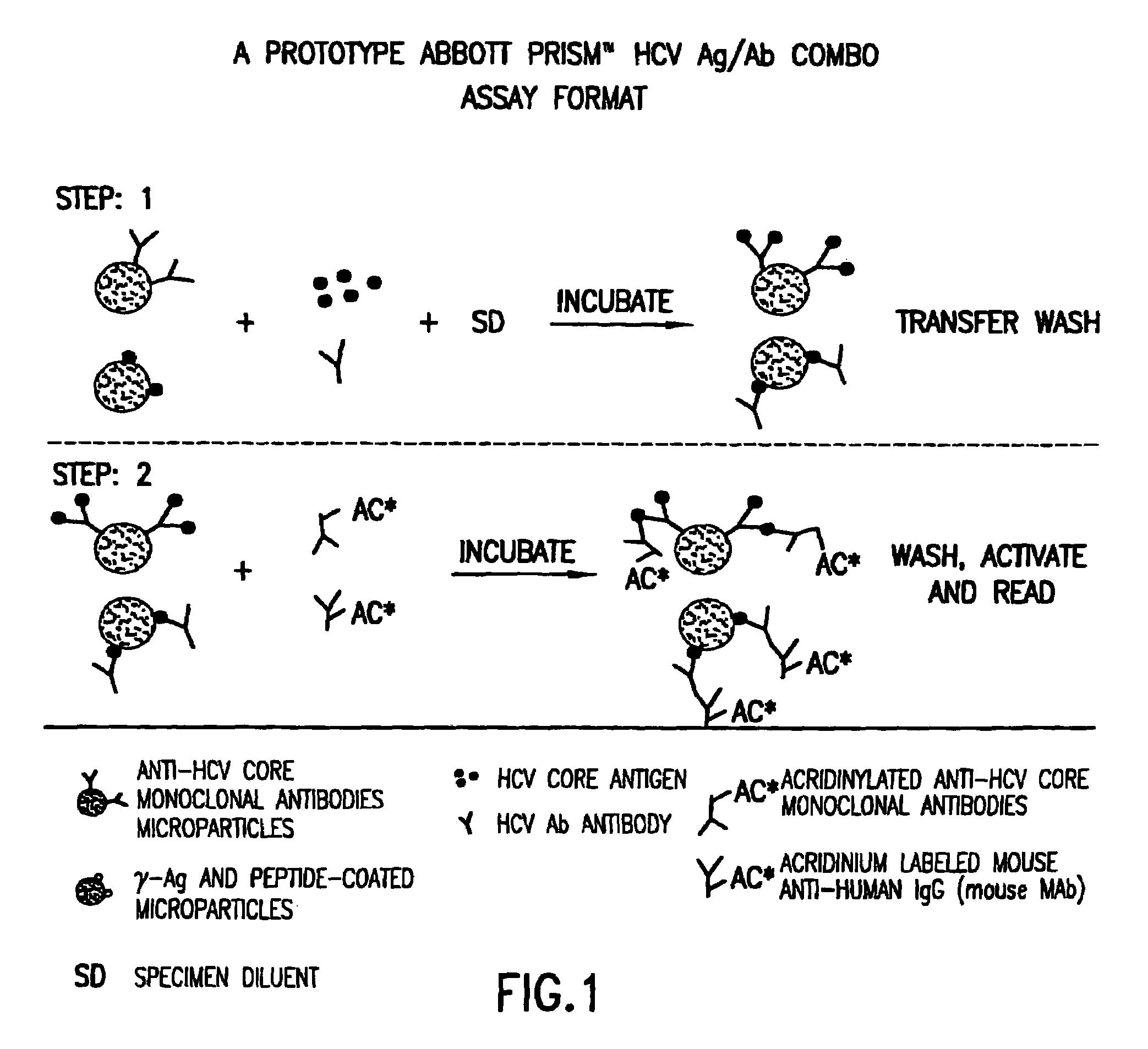
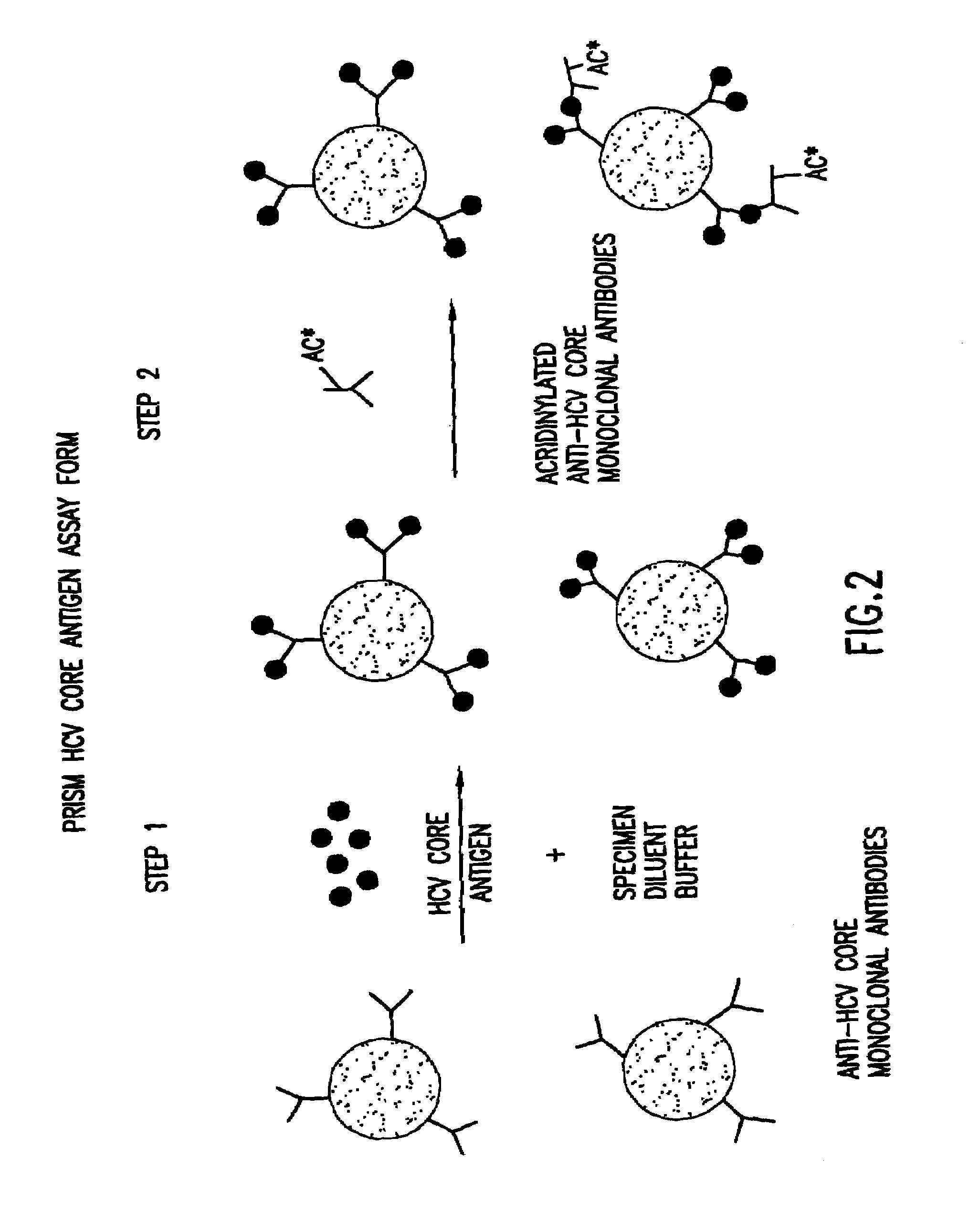
Methods for the simultaneous detection of HCV antigens and HCV antibodies
The subject invention relates to methods for the simultaneous detection of Hepatitis C Virus (HCV) antigens as well as antibodies produced in response to HCV antigens. Furthermore, the subject invention allows one to detect antigens in the early, acute stage of infection, even prior to the development of antibodies, thereby allowing for early detection of infected blood and blood products, thus improving the safety of the blood supply.
Owner:ABBOTT LAB INC
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
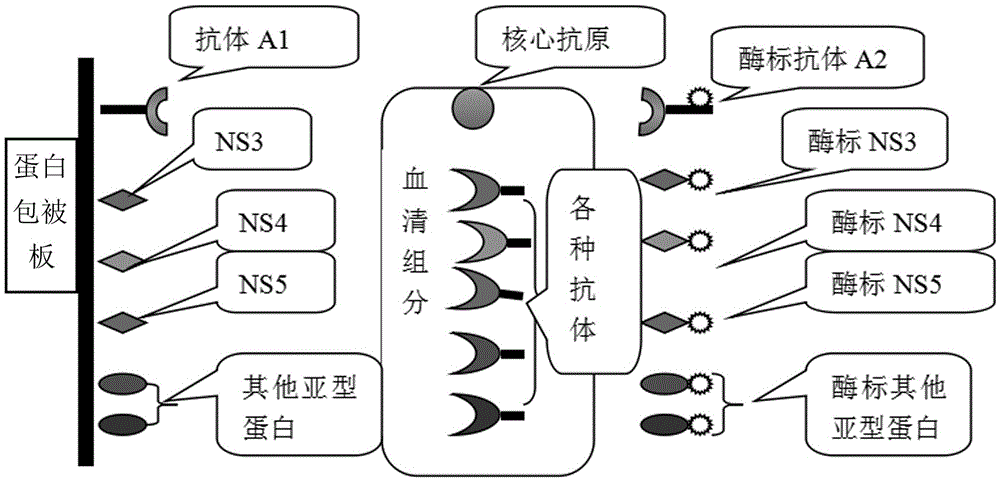
Disclosed are a hepatitis C virus antigen enzyme-linked immunoassay reagent box and a method for making the same. The invention obtains the cell strain of excretive anti HCV core antigen by analyzing the core antigen array of the hepatitis C virus different type and cloning the core antigen gene of HCV, purifying out the high activity monoclonal antibody with four core aa expression sites of HCV, wherein the Cab1 and Cabs are used as coating antibodies, the Cab3 and Cab4 are used as enzyme labeled antibodies; employing double antibodies sandwich technology to prepare HCV-cAg ELISA diagnosing reagent box.
Owner:湖南景达基因有限公司
Combined detection kit for hepatitis c virus (HCV) antigen-antibody
InactiveCN106093402AImprove specific recognition abilityReduce non-specific bindingMaterial analysisHCV AntibodyHcv core antigen
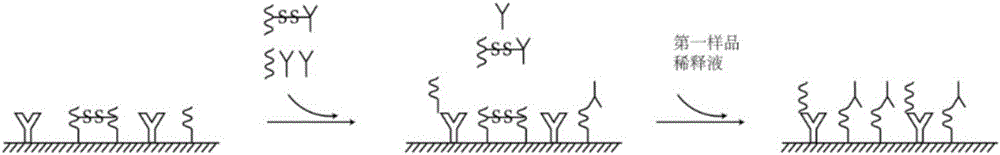
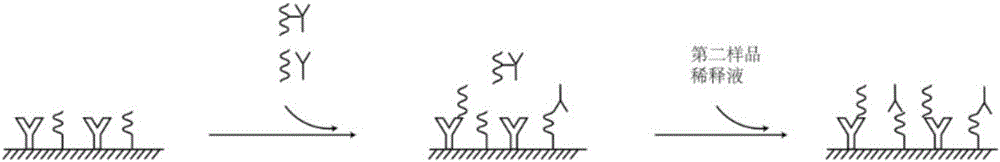
The invention discloses a combined detection kit for HCV antigen-antibody. The kit comprises a first monoclonal antibody against HCV core, a first HCV recombinant chimeric antigen, a first diluted sample solution, a second diluted sample solution, a first enzyme-labeled second monoclonal antibody against HCV core antigen, a first ligand-labeled second HCV recombinant chimeric antigen, a second enzyme-labeled second ligand, a developer and a stopping solution, wherein the first diluted sample solution comprises a reducing agent, a first surfactant and a first denaturant; and the second diluted sample solution comprises a second surfactant and a second denaturant. The combined detection kit for the HCV antigen-antibody opens mismatched disulfide bonds in coating antigen and in an antigen-antibody complex of a sample in virtue of the first diluted sample solution, and destroys HCV core antigen and HCV antibody compounds in virtue of the second diluted sample solution, so more antigen is released; and thus, detection sensitivity is improved.
Owner:HUNAN KANGRUN PHARMA
Recombinant vaccinia virus having hepatitis c virus gene
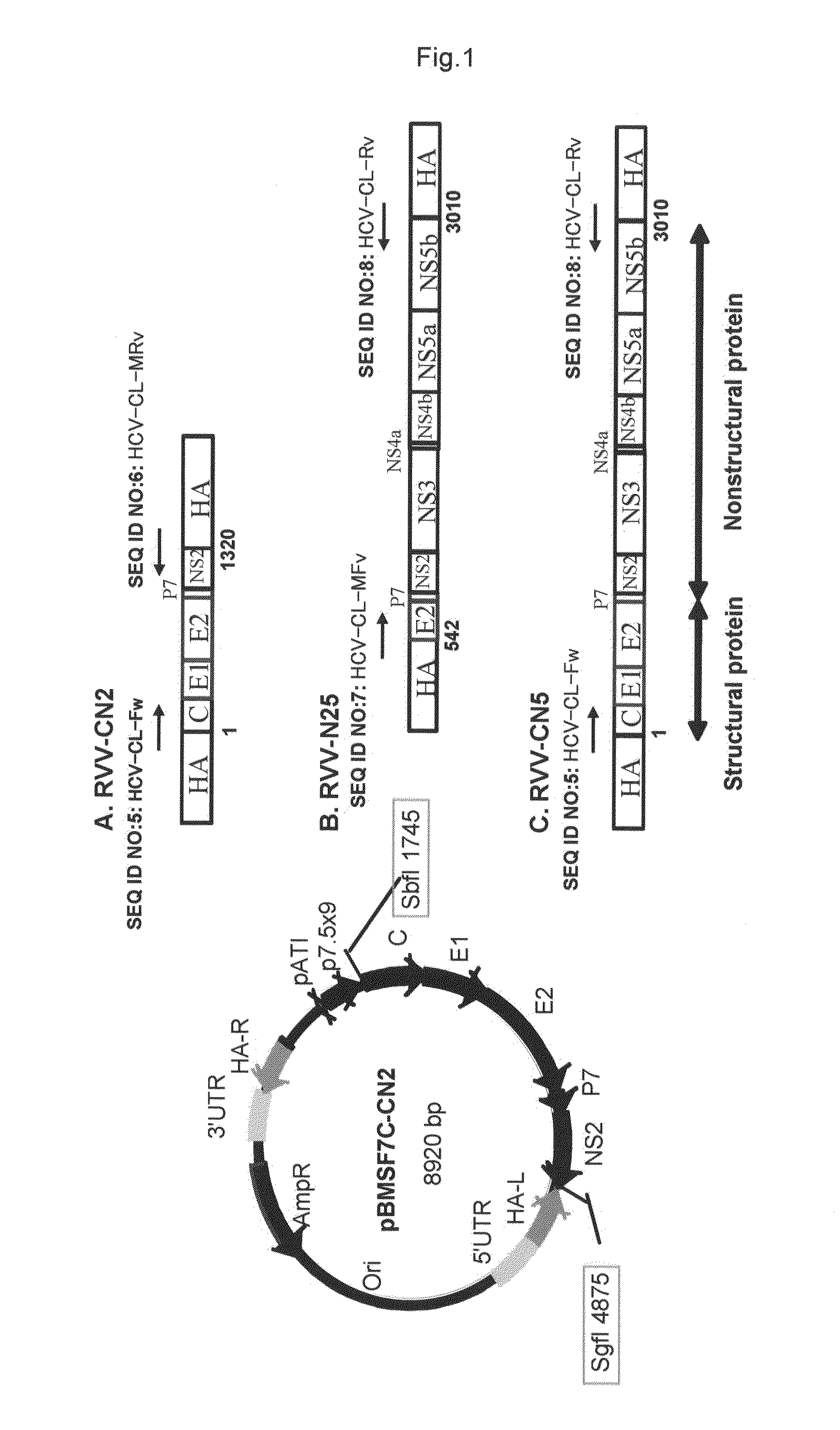
InactiveUS20110275139A1Potent hepatitis C infection prevention methodAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsHepacivirusGene
Provided is a recombinant virus which is efficacious in preventing the onset of hepatitis C infection and has a high safety. Also provided is a vaccine for hepatitis C virus which contains the recombinant virus. A recombinant vaccinia virus which can express hepatitis C virus gene. The hepatitis C virus vaccine as described above contains the recombinant virus as described above.
Owner:KM BIOLOGICS CO LTD +1
Combination hepatitis C virus antigen and antibody detection method
InactiveUS8865398B2Microbiological testing/measurementImmunoglobulins against virusesWhole blood productHepacivirus
An in vitro method that allows detection of hepatitis C by detecting hepatitis C virus (HCV) core protein and antibodies to HCV core protein (anti-core antibodies) in a single assay is provided. Cross-reactivity is eliminated in the method preferably by utilizing short peptides, each of which has an amino acid sequence that corresponds to an immunodominant region of the native core protein but which does not wholly encompass the epitope bound by the antibodies utilized in the method. The method can be used to detect the presence of HCV in a subject, and / or to determine the suitability of donor blood or blood products for transfusion purposes. Also provided are diagnostic kits for carrying out the method and a process for selecting suitable capture peptides and monoclonal antibodies for use in the combination method.
Owner:ABBOTT LAB INC
Kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence
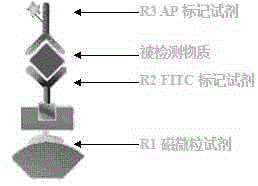
InactiveCN104914244AAvoid missing detectionGuaranteed specificityBiological material analysisEnzyme immunoassaysImmunoresponse
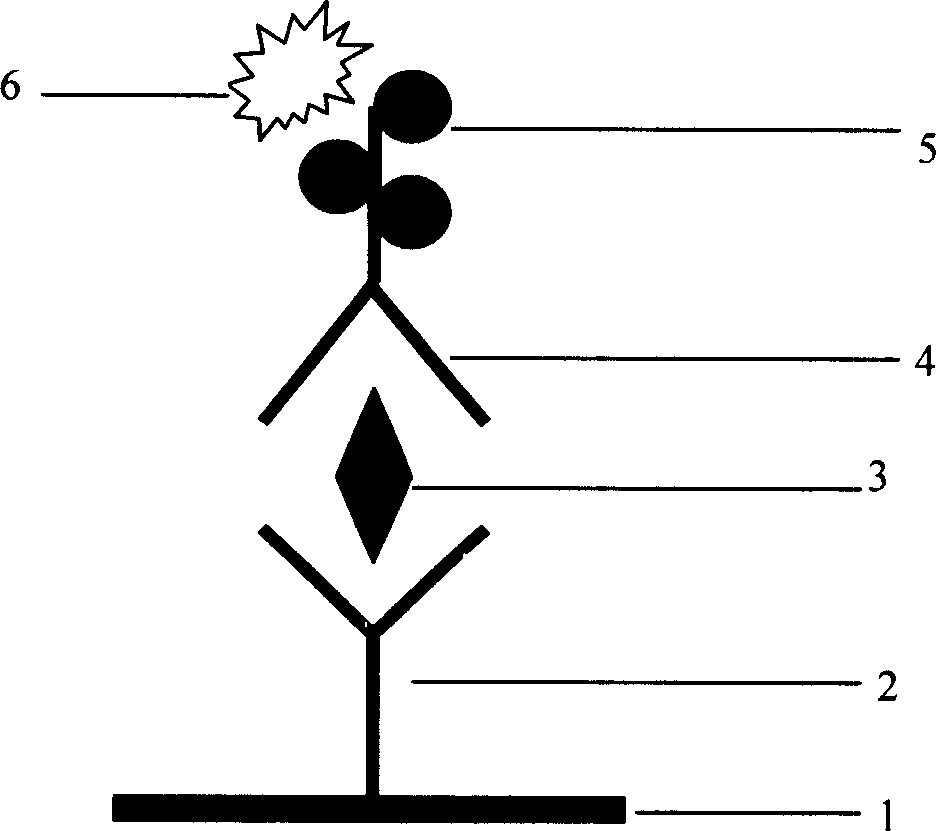
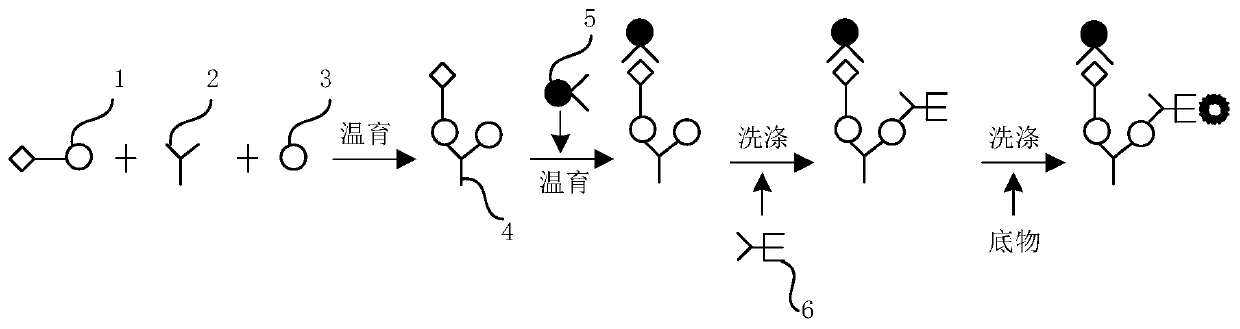
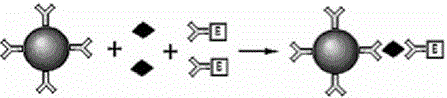
The invention provides a kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence. According to the invention, the principles of chemiluminescence are utilized; anti-FITC-coated magnetic particles are bonded with an FITC-coated reagent and then undergo immunoreaction with a detected object; after immunoreaction of an AP-labeled substance, an immunoreaction chain (as shown in a figure 1 which is described in the specification) is constructed; luminous sensitivity of a substrate catalyzed by AP is far higher than enzyme immunoassay development, so reaction sensitivity is enhanced; meanwhile, FITC is used to simultaneously marking antigen and antibody, so hepatitis C virus antibody and core antigen are detected at the same time. The kit provided by the invention can more accurately detect hepatitis C, is free of leak detection, achieves the effect of early discovery and has a high application value in prevention of hepatitis C.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Preparation method of hepatitis c virus antigen and antibody combined detection reagent, and detection card
PendingCN110133269AAvoid mutual interferenceImprove accuracyMaterial analysisAntigenHepatitis C Virus Antigen
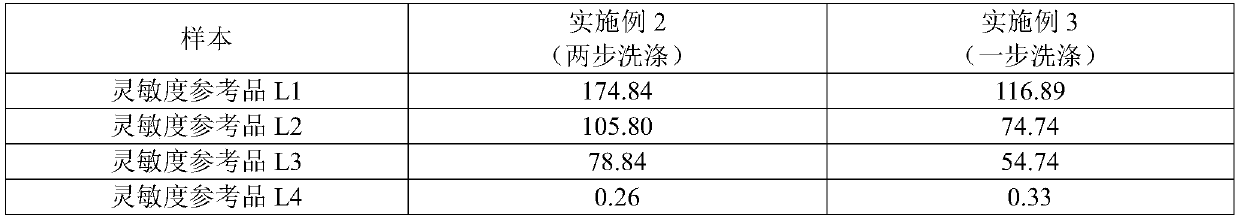
The invention relates to the field of hepatitis c virus detection, and discloses a preparation method of a hepatitis c virus antigen and antibody combined detection reagent, and a detection card. A double antibody and double antigen sandwich method is used for detecting hepatitis c virus antigen and antibody, a to-be-detected sample is mixed with a sample diluent, a HCV antibody and a HCV core antigen in the sample respectively react with a biotin-labeled antigen and a biotin-labeled antibody, then, the mixed sample is added in a test strip one time, thus, combined detection for the HCV antigen and the HCV antibody is realized. With the method and the detection card provided by the invention, mutual interference between the HCV antigen and the HCV antibody is avoided, and HCV antigen positive, HCV antibody positive or both positive can be distinguished, accuracy and sensitivity of the HCV detection are improved, and the method and the detection card can be used for early screening andclinical auxiliary diagnosis of the HCV so as to remedy missing detection of a HCV antibody diagnosis kit in an infection 'window period', can realize a complementary effect of the HCV antibody detection and the HCV antigen detection, not only improve working efficiency, but also save cost.
Owner:HUNAN KANGRUN PHARMA
Reagent for high throughput combined detection of hepatitis c virus antigen-antibody
ActiveCN105004862AIntuitive identification of infection periodHigh sensitivityBiological testingFluorescence/phosphorescenceAntigenFluorescein
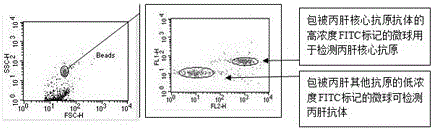
The invention provides a reagent for high throughput combined detection of hepatitis c virus antigen-antibody. By the flow cytometry theory, different concentrations of FITC fluorescein-labeled microspheres are correspondingly coated with antibody and antigen; after addition of a detection sample, the microspheres, the detection sample and the PE-labeled paired antibody and antigen form a sandwich structure; and under the action of 480nm exciting light, the effects of simultaneous detection of hepatitis c virus antigen-antibody can be achieved in a high throughput way according to different emitted light intensities of fluoresceins with different concentrations. By the utilization of fluorescent color-developing effect, reaction sensitivity is enhanced, and hepatitis c virus antibody and core antigen can be detected simultaneously by the utilization of FITC-labeled microspheres for simultaneous detection of antigen-antibody. The reagent is more accurate in hepatitis c detection, has no defect in detection leakage, achieves the early detection effect and has high application value in prevention of hepatitis c.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Newly found hepatitis C virus epitope
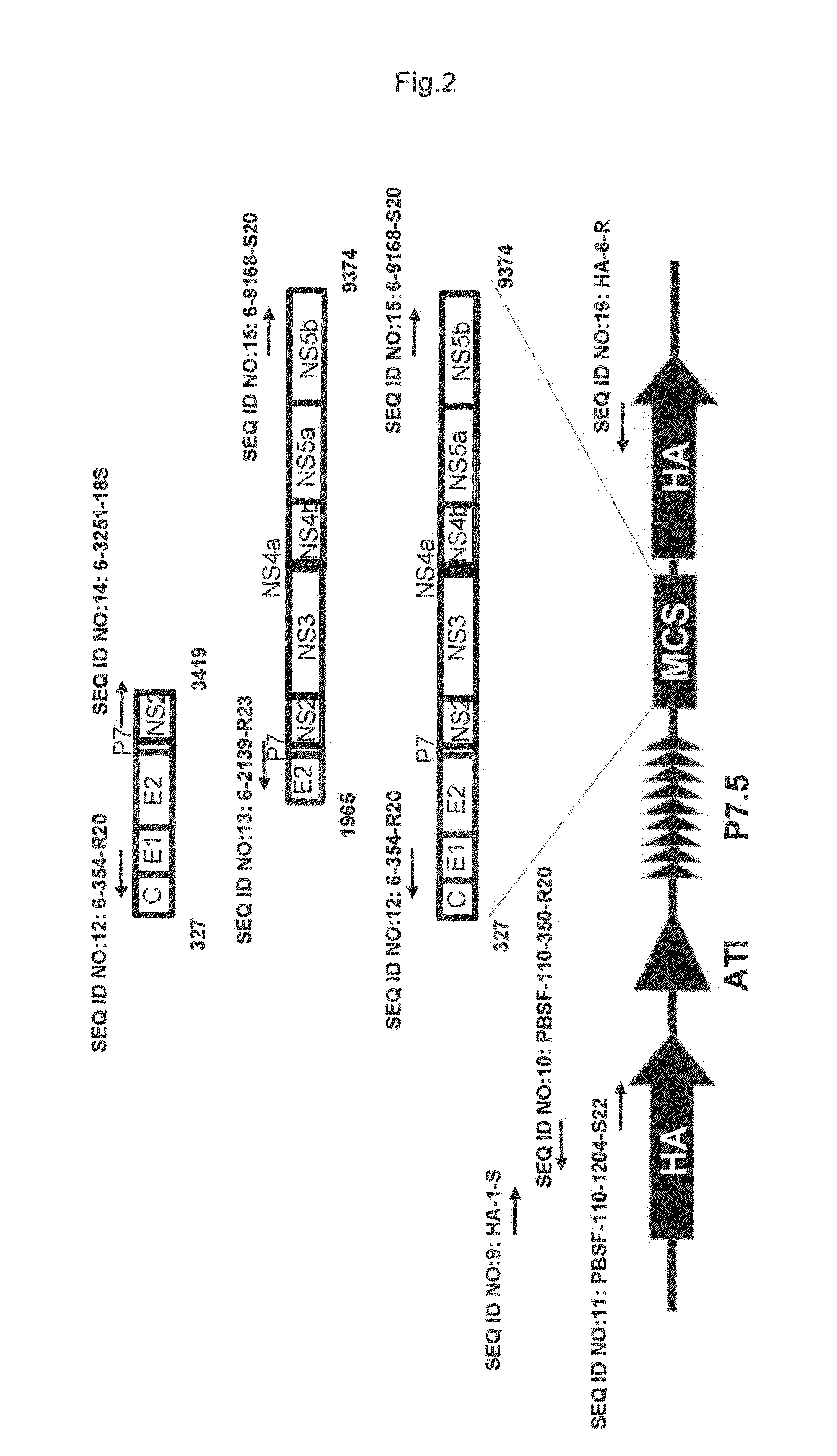
The invention relates to the technology of bioinformatics and immunology and provides a newly found hepatitis C virus epitope. The sequence of the hepatitis C virus epitope is an epitope polypeptide P7(774-782)AAWYIKGRL. The invention also relates to the application of the epitope polypeptide to the preparation of medicaments for treating hepatitis C virus and to the preparation of hepatitis C virus vaccines. In-vivo and in-vitro experiments show that that the epitope has immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Hepatitis C virus vaccine composition
InactiveCN102596244AAvoid infectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenAdjuvant
Disclosed is an effective HCV vaccine composition, which is developed as a result of the finding of an optimum combination of an HCV antigen capable of inducing an antibody having an inhibitory activity on the infection by an HCV and an adjuvant. Specifically disclosed is a hepatitis C virus vaccine composition which comprises: inactivated virus particles produced by inactivating infectious hepatitis C virus particles that are produced from hepatitis C virus genome containing sequences respectively encoding NS3 protein, NS4A protein, NS4B protein, NS5A protein and NS5B protein derived from hepatitis C virus strain JFH1; a non-methylated CpG-containing oligonucleotide represented by SEQ ID NO:5 shown in the Sequence Listing; and aluminum hydroxide.
Owner:TORAY IND INC +2
Compositions and methods for the treatment of hepatitis c
The present invention provides compositions and methods for delivery of one or more hepatitis C virus (HCV) antigens using a bacterium recombinantly encoding and expressing such antigens. In certain embodiments, the bacterial platform comprises the use of attenuated and killed but metabolically active forms of Listeria monocytogenes.
Owner:ADURO BIOTECH
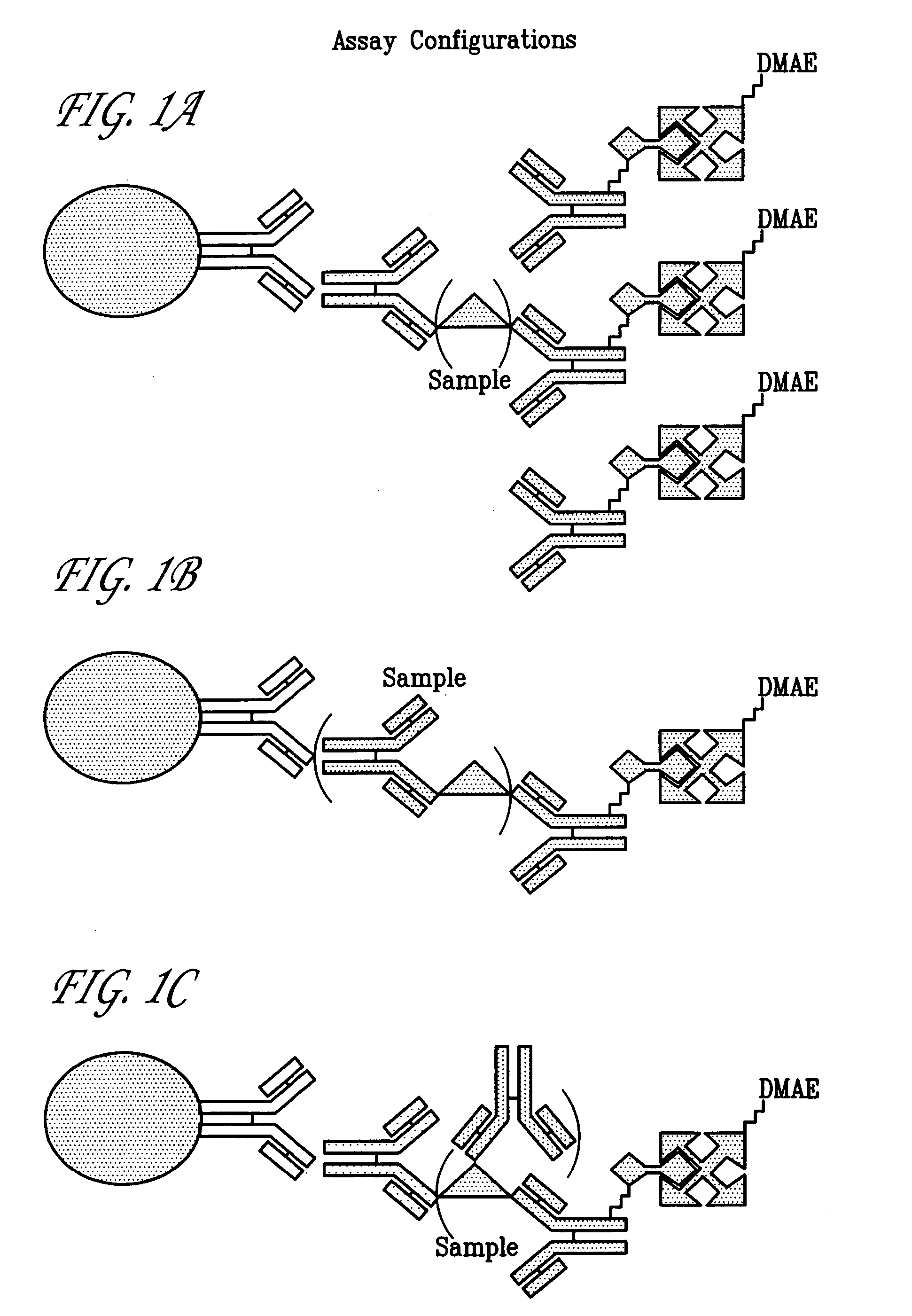
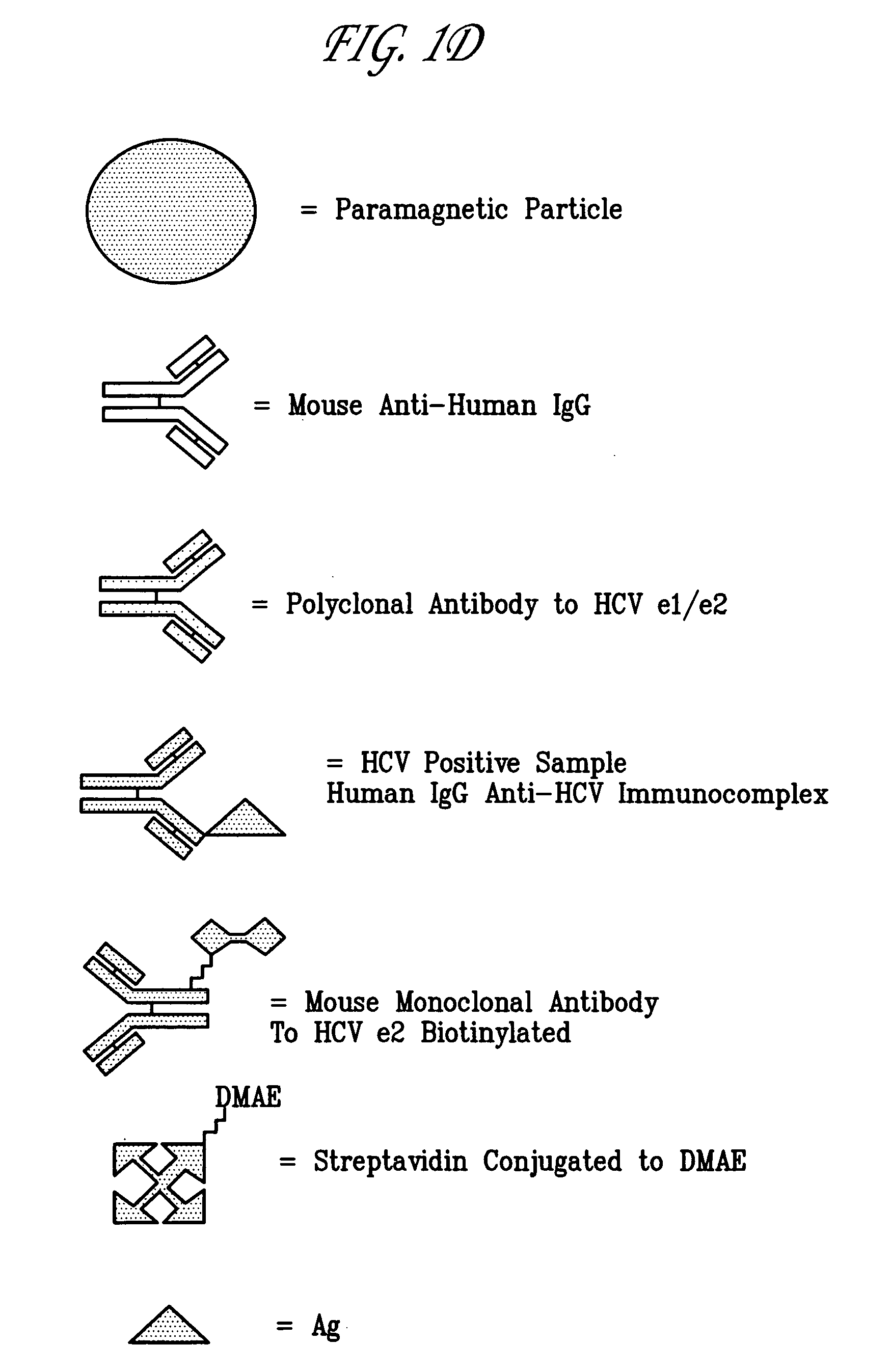
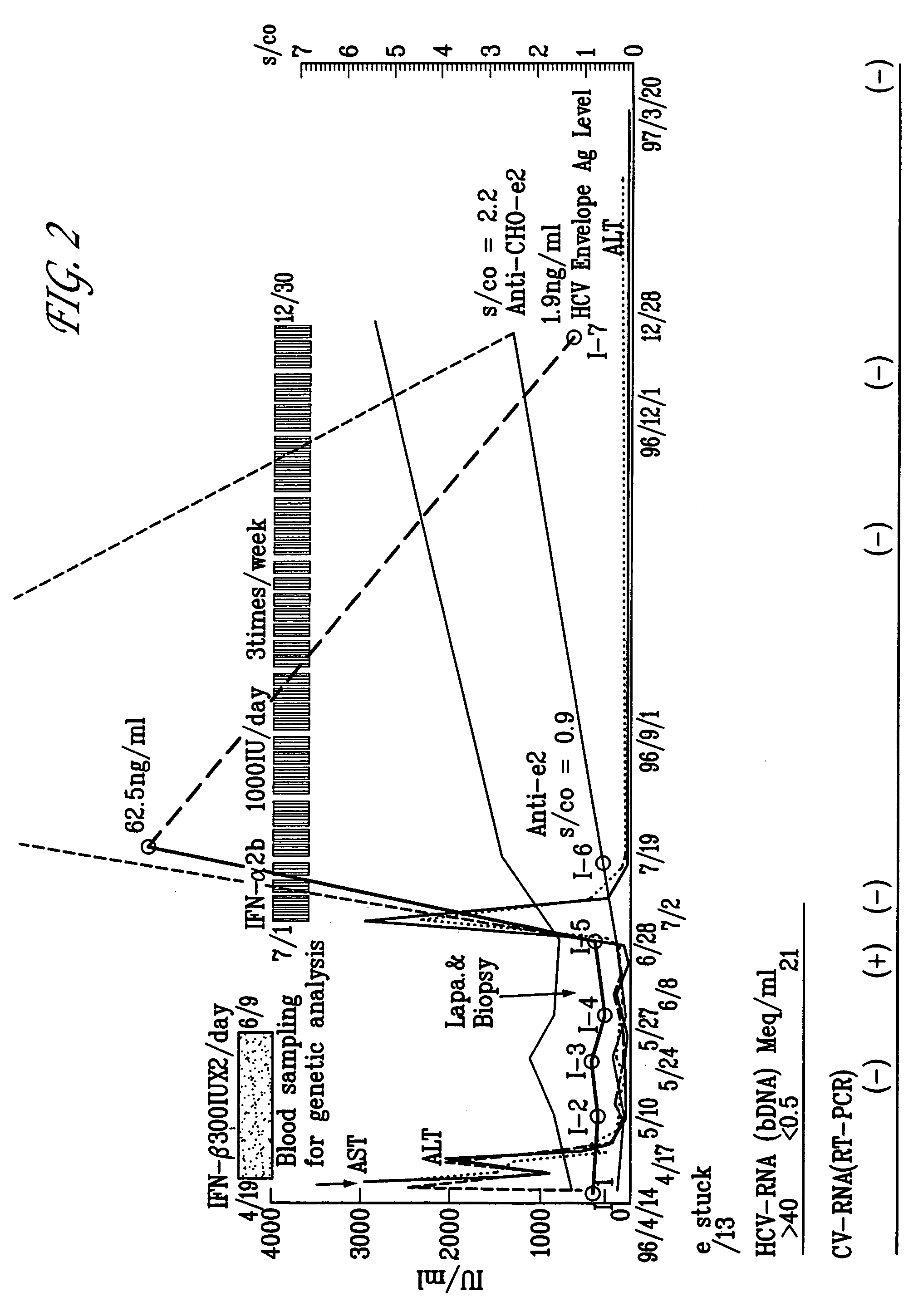
Hepatitis C viral antigen immunoassay detection systems
InactiveUS7108967B2Microbiological testing/measurementBiological material analysisWhole blood productImmune complex deposition
Owner:GRIFOLS WORLDWIDE OPERATIONS
Hepatitis C virus antibody detection kit, preparation method and detection method
PendingCN111579781AFully combinedHigh detection sensitivityChemiluminescene/bioluminescenceAntibody combining siteBinding site
The invention relates to the technical field of in-vitro diagnosis and detection, in particular to a hepatitis C virus antibody kit, a preparation method and a detection method. The hepatitis C virusantibody kit comprises a reagent R1, a reagent R2 and a reagent R3, and the reagent R1 comprises a streptavidin magnetic particle solution; the R2 reagent comprises a mixed reagent of a biotinylated hepatitis C virus antigen and a hepatitis C virus recombinant antigen; and the R3 reagent comprises an alkaline phosphatase labeled hepatitis C virus monoclonal antibody solution. According to the invention, the hepatitis C virus antibody, the biotinylated hepatitis C virus antigen and the hepatitis C virus recombinant antigen form a 'sandwich' sandwich compound, so that the blocking of antigen andantibody binding sites due to the addition of magnetic particles and enzyme markers is avoided, and the sensitivity and specificity of the detection of the hepatitis C virus antibody are remarkably improved.
Owner:深圳市爱康试剂有限公司
HCV antigen detection board and method for detecting HCV antigen by using same
InactiveCN102043048AStrong specificitySimple and fast operationColor/spectral properties measurementsBiotin-streptavidin complexLong arm
The invention provides a hepatitis C virus (HCV) antigen detection board and a method for detecting an HCV antigen by using the same. The method comprises the following steps of: diluting a purified HCV resistant polyclonal antibody until the concentration is 5 to 50mu g / ml by the conventional method; adding 50 to 200mu l of polyclonal antibody into each hole of a polystyrene board; standing overnight at the temperature of 4 DEG C; washing the board; adding 100 to 300mu l of the conventional bovine serum albumin into each hole of the polystyrene board and sealing the detection board; standing overnight at the temperature of between 2 and 8 DEG C; washing the board and drying in the air; storing at the temperature of between 2 and 8 DEG C to obtain the HCV antigen detection board; and detecting the HCV antigen through the HCV antigen detection board by using long arm biotin labeled monoclonal (polyclonal) HCV-immunoglobulin G (IgG), horse radish peroxidase labeled streptavidin or horse radish peroxidase labeled monoclonal (polyclonal) HCV-IgG as an amplification system. Through the method, detection processes can be reduced, detection time can be shortened, a background can be lowered by lowering a cross reaction and detection sensitivity can be improved; and the method for detecting the HCV antigen has high specificity and sensitivity, and is conveniently and quickly operated; and a reliable technical path is provided for early diagnosis, large-scale quick screening, quantitative detection and the like of HCV infection.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Immunotherapy of virus infection
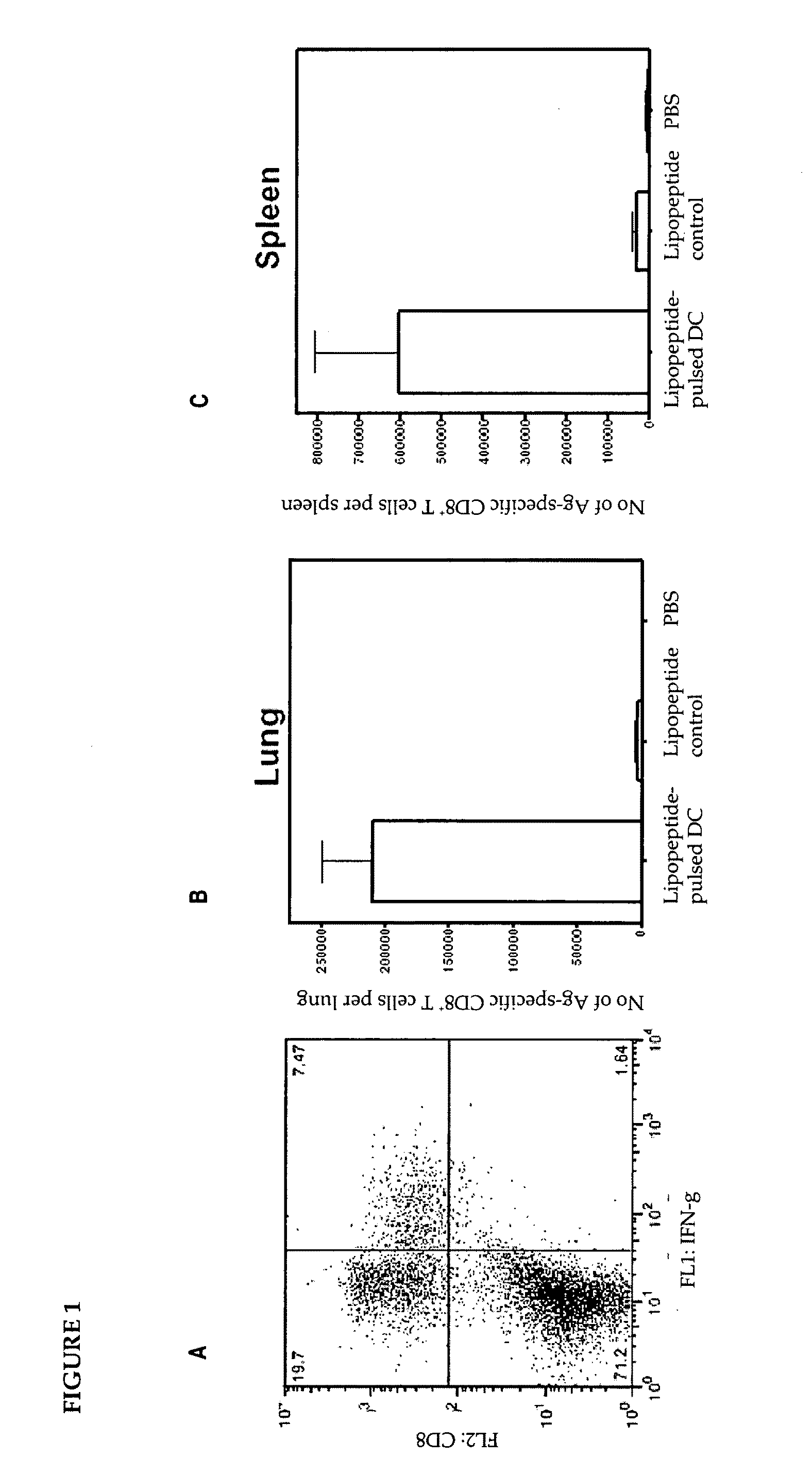
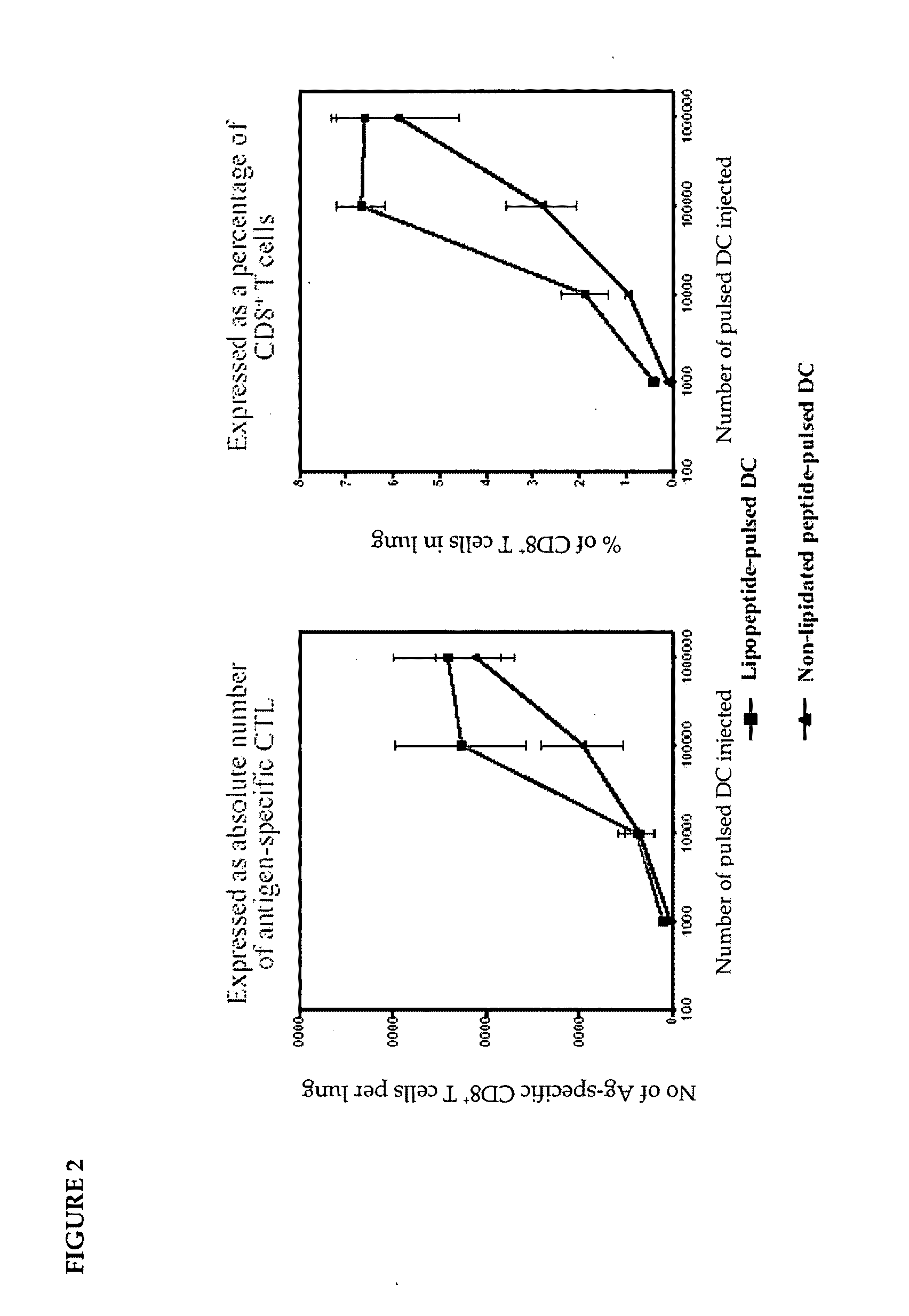
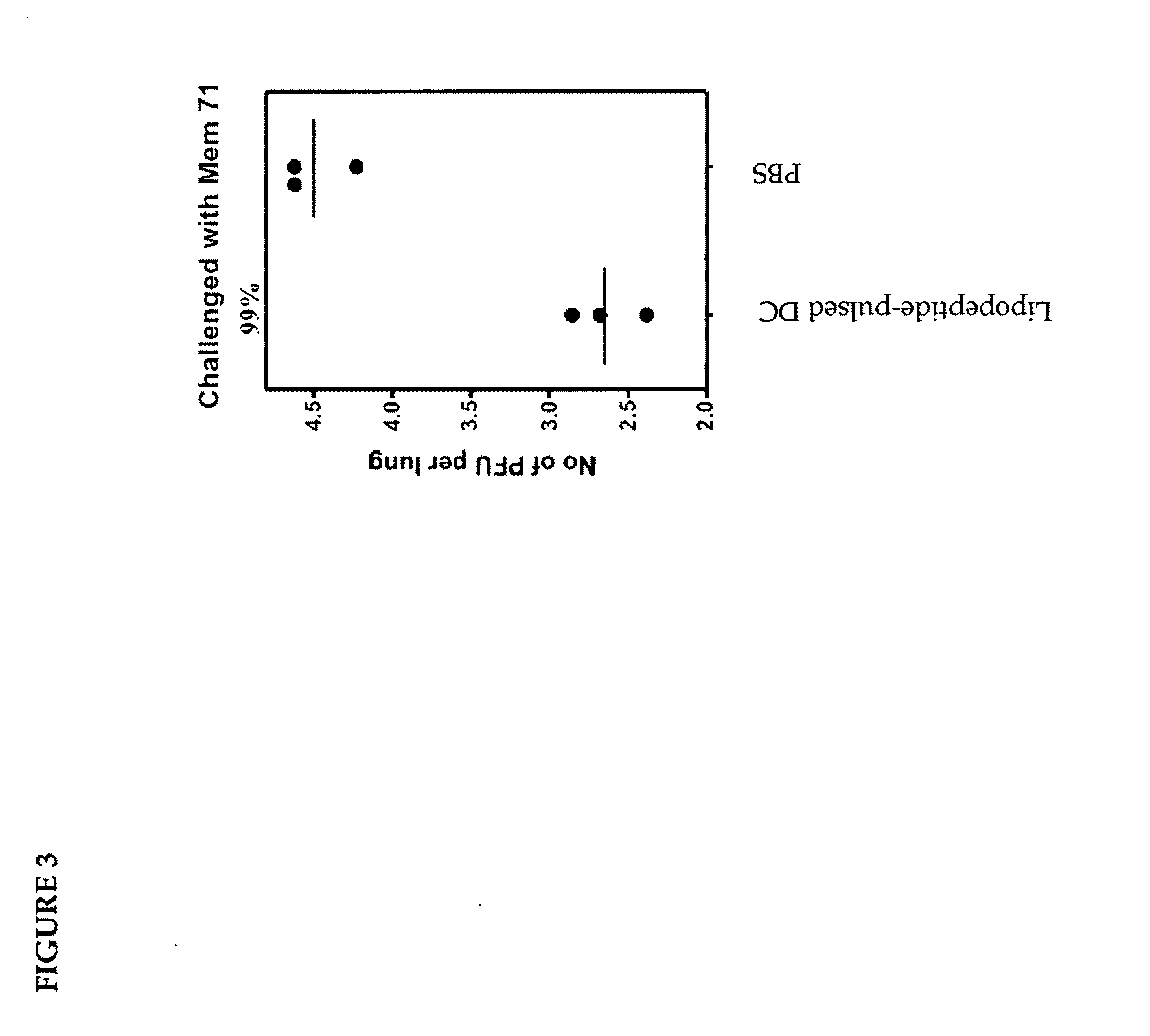
The present invention relates to a method of treating or preventing a virus infection in a subject. In particular, it relates to the use of autologous dendritic cells that have been matured and loaded ex vivo with hepatitis C virus (HCV) antigens, to initiate a cellular immune response in HCV-positive patients, after autologous transfusion.
Owner:THE MACFARLANE BURNET INSTITUTE FOR MEDICAL RESEARCH AND PUBLIC HEALTH LTD
Method and device for detecting viral antigen of C type hepatitis
The invention discloses a method and device for detecting virus antigen of type-C hepatitis. The invention designs a compound antigen for type-C hepatitis virus made up of ten specificity antigens in epi-position, the reagent for detecting the type-C hepatitis virus antigen constructed by the inducer and by using the antigens, it can identifies the type-C hepatitis antigen in serum and plasma infected by the type-C hepatitis virus through the detection. The reagent box has a high specificity and low false negative rate and false positive rate.
Owner:KUNMING KELAISEN BIOLOGICAL TECH
Hepatitis C virus (HCV) antigen and antibody combined detection kit
The invention discloses a hepatitis C virus (HCV) antigen and antibody combined detection kit. The kit comprises magnetic particles, a sample diluent, a negative control, an antigen positive control,an antibody positive control and an enzyme conjugate, the magnetic particles are coated with recombinant HCV virus antigens and mouse anti-human HCV core antigen monoclonal antibodies; the antibody positive control contains HCV antibody positive human plasma; and the antigen positive control contains HCV recombinant core antigens. According to the kit, the chemiluminescence and magnetic particle separation technologies are combined, and the HCV recombinant core antigens and the HCV antibodies in serum are detected at the same time, by matching with a full-automatic chemiluminiscence instrumentof the Zhengzhou Antu Bio-Engineering Co.,Ltd, and can be used as a primary screening test for clinical detection, so that the window period is further shortened, and the HCV propagation is effectively controlled.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Hepatitis C virus antigen-antibody joint detection method and kit
The invention relates to a hepatitis C virus antigen-antibody joint detection method in the technical field of immunodetection. The kit comprises at least two detection areas, whether a first compoundformed by a donor-hepatitis C virus antibody-receptor exists or not is detected in one detection area, and whether a second compound formed by a donor-hepatitis C virus antigen-receptor exists or notis detected in the other detection area, a donor can generate active oxygen in an excited state, and a receptor can react with the active oxygen to generate a detectable chemiluminescence signal. Themethod is advantaged in that the HCV core antigen and the antibody are jointly detected, so detection accuracy is improved, detection cost is low, moreover, a treating agent is added into a core antigen detection hole, so interference of a low-affinity antibody in an early body is reduced, the antigen detection sensitivity in a conversion period is improved, and the HCV detection sensitivity is further improved.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
A kind of hepatitis C virus antigen-antibody combined detection kit and preparation method thereof
A hepatitis c virus antigen-antibody joint detection reagent box is characterized by comprising a calibrator(1), a double-marker enzyme conjugate (2), a negative and positive contrast (3), light-emitting liquid (4) and a micropore coated plate (5), wherein the light-emitting liquid contains light-emitting liquid 1 and light-emitting liquid 2, the light-emitting liquid 1 contains luminal 0.7 g / L, cinnamic acid 0.9 g / L, 4-iodophenylboronic acid 0.2 g / L, iodobiphenol 0.25 g / L, dimethylformamide 25 ml / L, polyving akohol 5 g / L, polyvinylpyrrolidone 8 g / L, polyethylene glycol 600 3 g / L, ethylenediamine tetraacetic acid 4 g / L, gentamicin sulfate 1600 thousands / L, urea peroxide 0.4 g / L, and pH 9.0 Tris buffer solution 0.1 mol / L. The light-emitting liquid 2 contains acridinium ester derivative 0.1 mg / ml, polyethylene glycol 600 3 g / L and 0.1 mol / L of pH 9.0 Tris buffer solution containing 0.1% of TWEEN-20. The invention further discloses a preparation method and a using method of the reagent box. The hepatitis c virus antigen-antibody joint detection reagent box has the advantages of being quick in reaction and low in cost.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Hepatitis C virus antigen fluorescent immunochromatography detection kit and preparation method therefor
PendingCN109324184ARapid Quantitative DetectionConvenient Quantitative DetectionMaterial analysisDiluentCapture antibody
The invention discloses a hepatitis C virus antigen fluorescent immunochromatography detection kit and a preparation method therefor. The detection kit comprises the following components: a fluorescent microsphere labeled hepatitis C virus antibody 1, a hepatitis C virus antibody 2, a goat anti-mouse capture antibody, a phosphate buffer, and a sample diluent. The preparation method for the detection kit comprises the following steps: preparing the sample diluent; preparing the fluorescent microsphere labeled hepatitis C virus antibody 1; preparing a carrier 1 coated with the fluorescent microsphere labeled hepatitis C virus antibody 1; preparing a carrier 2 coated with the hepatitis C virus antibody 2 and the goat anti-mouse capture antibody; and assembling the standard substance to obtainthe fluorescent immunochromatography detection kit. The detection kit can rapidly and conveniently carry out quantitative detection on hepatitis C virus antigens on site, and has good detection sensitivity, precision and accuracy.
Owner:XIAMEN TONGRENXIN BIO-TECH CO LTD
Hepatitis C virus antigen-antibody joint inspection kit and application thereof
PendingCN111521780AReduced detection windowReduce distractionsMaterial analysisReceptorAntigen testing
The invention relates to a hepatitis C virus antigen-antibody joint detection kit and an application thereof, belongs to the technical field of immunodetection. The kit comprises the following components, a receptor bound with a first antigen, a second antigen, a receptor binding to the first antibody, and a second antibody, wherein the first antigen and the second antigen can be specifically combined with a variable region of a hepatitis C virus antibody, the first antibody and the second antibody can be specifically combined with different epitopes of a hepatitis C virus antigen. The kit isadvantaged in that the HCV antigen-antibody joint detection kit can be used for joint detection of HCV core antigen and antibody, so a detection window period is shortened, detection cost is low, moreover, the kit further comprises a treating agent, and the treating agent can reduce the interference of a low-affinity antibody in an early body and improve the antigen detection sensitivity in a conversion period, so the HCV detection sensitivity is improved, and the HCV detection window period is further shortened.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Hepatitis C virus antibody detection kit and application thereof
InactiveCN113189348AStrong specificityGood repeatabilityChemiluminescene/bioluminescenceBiological testingAntigenEnzyme binding
The invention discloses a hepatitis C virus antibody detection kit and application thereof. The hepatitis C virus antibody detection kit comprises a hepatitis C virus antigen coated plate, an enzyme conjugate and a chemiluminescent substrate. The prepared kit is used for detecting the antibody and has the effects of high specificity, good repeatability and high sensitivity.
Owner:中山生物工程有限公司
A pair of monoclonal antibodies capable of specifically recognizing hcv NS3 protein and its application
ActiveCN109678954BHigh sensitivityImprove featuresSsRNA viruses positive-senseVirus peptidesAntigenAmino acid
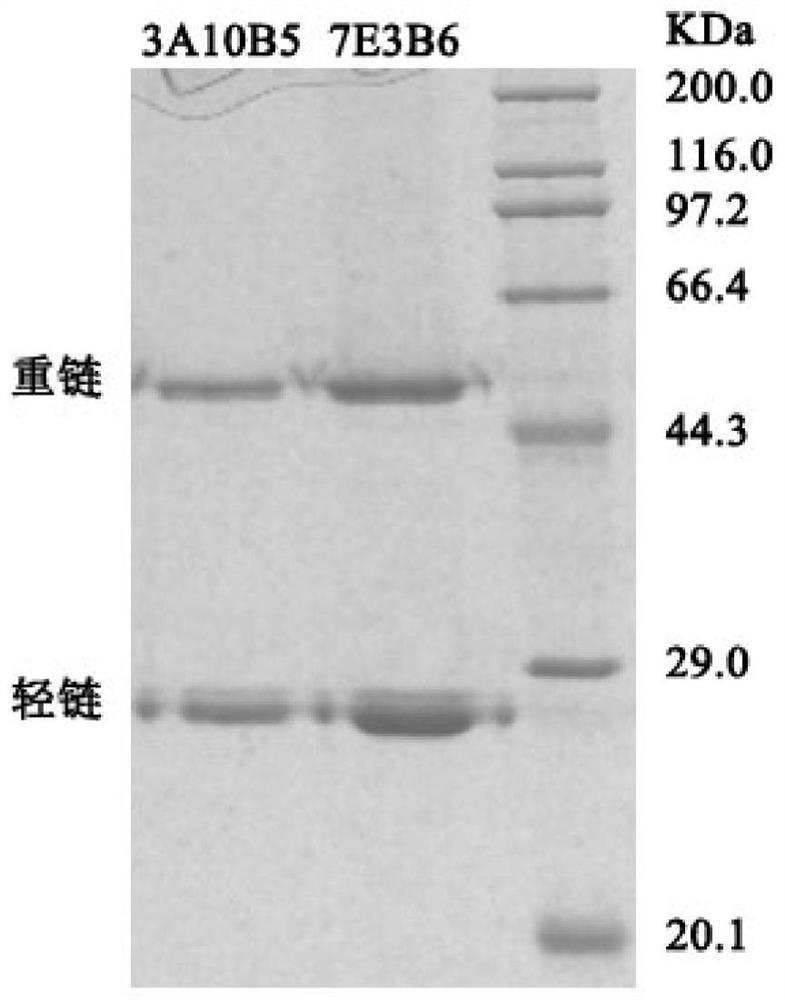
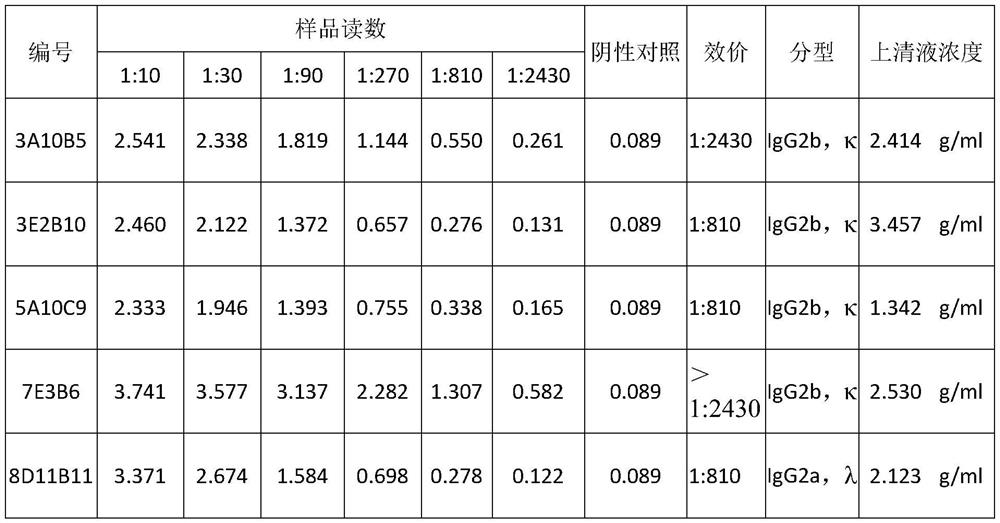
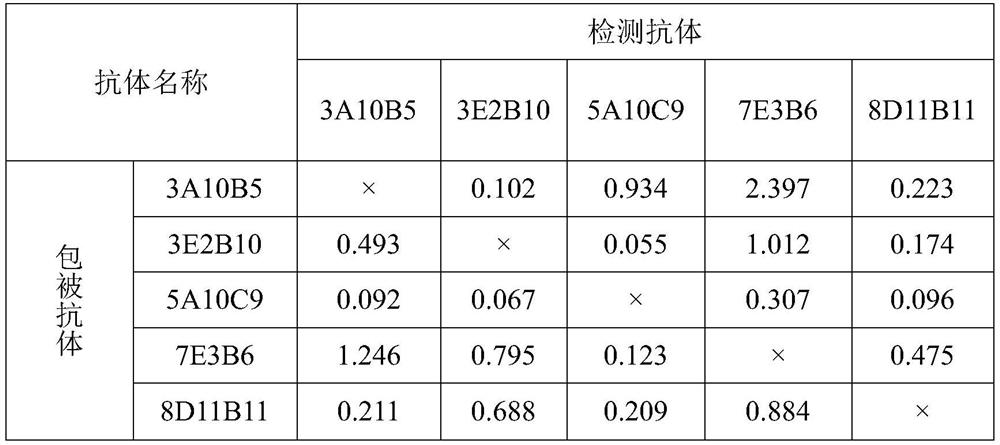
The invention discloses two monoclonal antibodies capable of specifically recognizing HCV NS3 protein used in pairs, wherein the antibody is named 3A10B5, the amino acid sequence of its light chain is shown in SEQ ID NO.1, and the amino acid sequence of its heavy chain is As shown in SEQ ID NO.2; another antibody named 7E3B6, the amino acid sequence of its light chain is shown in SEQ ID NO.3, and the amino acid sequence of its heavy chain is shown in SEQ ID NO.4. The invention also discloses the application of the monoclonal antibody as a coating antibody and a detection antibody paired in the preparation of a hepatitis C virus antigen ELISA detection reagent or kit. Experiments have proved that the binding of the monoclonal antibody of the present invention to the NS3 antigen in the serum of HCV infected patients has high specificity and affinity, and has important application value for the screening of hepatitis C virus.
Owner:山东莱博生物科技有限公司
A kind of hepatitis C virus antigen-antibody combined detection kit
ActiveCN104407143BPotential clinical application value is goodReduce missed detection rateMaterial analysisAntigenHepatitis C virus core
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
A high-throughput combined detection reagent for hepatitis C virus antigen antibody
ActiveCN105004862BAvoid missing detectionGuaranteed specificityBiological testingFluorescence/phosphorescenceMicrosphereFluorescein
Disclosed is a reagent for high throughput combined detection of a hepatitis C virus antigen and antibody. Using the flow cytometry principle, different concentrations of FITC fluorescein-labeled microspheres correspond to coated antibodies and antigens; after addition of a detection sample, the microspheres, the detection sample and a PE-labeled paired antibody and antigen form a sandwich structure; and under the action of 480nm exciting light, the effects of simultaneous detection of a hepatitis C virus antigen and antibody can be achieved in a high throughput way according to different emitted light intensities of fluoresceins at different concentrations. By means of the utilization of the fluorescent coloration effect, the reaction sensitivity is enhanced, and a hepatitis C virus antibody and core antigen can be detected simultaneously by the means of utilization of FITC-labeled microspheres for simultaneous detection of the antigen and antibody.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com