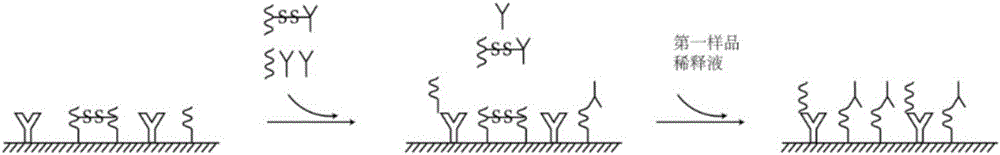
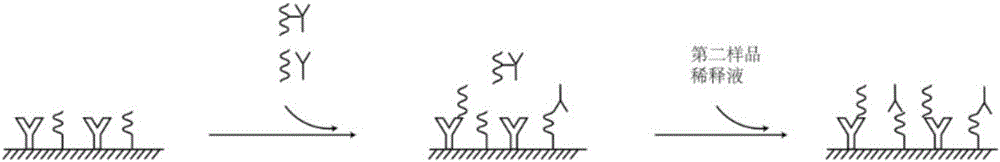
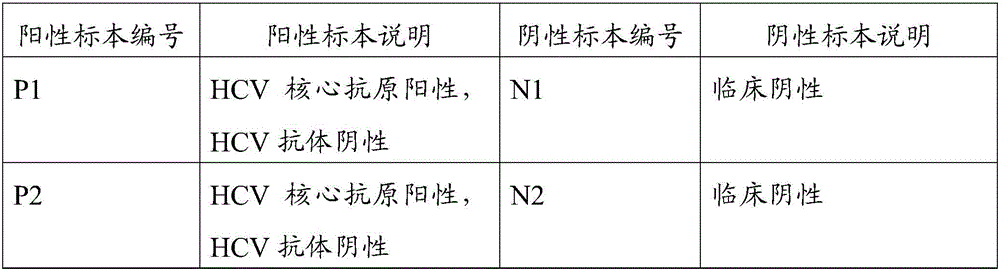
Combined detection kit for hepatitis c virus (HCV) antigen-antibody
A hepatitis C virus, antigen-antibody technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of inability to detect HCV antibodies, missed detection, and missed antibody detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The first sample dilution and the second sample dilution were prepared with phosphate buffer, and the specific components were as follows.
[0077] First sample dilution: 0.5 wt% β-mercaptoethanol, 0.05 wt% sodium dodecylsulfonate, 0.03 wt% Tween-20.
[0078] Second sample diluent: 3wt% sodium lauryl sarcosine and 3mol / L urea.
[0079] Dilute AB1 with carbonate buffer to a concentration of 3 μg / mL, dilute AG1 with carbonate buffer to a concentration of 6 μg / mL, and mix the two at a volume ratio of 1:1 to obtain a coating solution.
[0080] Then add the coating solution into the polystyrene microwell plate, and complete the coating after adsorption, washing, sealing and drying.
[0081] Add 100 μL of sample / positive control / negative control and 50 μL of the first sample dilution to each well, incubate at 37°C for 30 minutes without washing the plate, then add 50 μL of the second sample dilution to each well, incubate at 37°C for 30 minutes and wash the plate 6 times.
...
Embodiment 2
[0085] The first sample dilution and the second sample dilution were prepared with phosphate buffer, and the specific components were as follows.
[0086] The first sample dilution: 0.3wt% dithiothreitol, 0.01wt% sodium dodecylsulfonate, 0.01wt% guanidine hydrochloride.
[0087] The second sample diluent: 1 wt% dodecyl dimethyl betaine and 5 mol / L urea.
[0088] Dilute AB1 with carbonate buffer to a concentration of 3 μg / mL, dilute AG1 with carbonate buffer to a concentration of 6 μg / mL, and mix the two at a volume ratio of 1:1 to obtain a coating solution.
[0089] Then add the coating solution into the polystyrene microwell plate, and complete the coating after adsorption, washing, sealing and drying.
[0090] Add 100 μL of sample / positive control / negative control and 50 μL of the first sample dilution to each well, incubate at 37°C for 30 minutes without washing the plate, then add 50 μL of the second sample dilution to each well, incubate at 37°C for 30 minutes and wash t...
Embodiment 3
[0094] The first sample dilution and the second sample dilution were prepared with phosphate buffer, and the specific components were as follows.
[0095] First sample dilution: 1 wt% cysteine, 0.08 wt% sodium dodecylsulfonate, 0.1 wt% guanidine hydrochloride.
[0096] The second sample diluent: 5 wt% dodecyl dimethyl betaine and 1 mol / L polyethylene glycol octyl phenyl ether.
[0097] Dilute AB1 with carbonate buffer to a concentration of 3 μg / mL, dilute AG1 with carbonate buffer to a concentration of 6 μg / mL, and mix the two at a volume ratio of 1:1 to obtain a coating solution.
[0098] Then add the coating solution into the polystyrene microwell plate, and complete the coating after adsorption, washing, sealing and drying.
[0099] Add 100 μL of sample / positive control / negative control and 50 μL of the first sample dilution to each well, incubate at 37°C for 30 minutes without washing the plate, then add 50 μL of the second sample dilution to each well, incubate at 37°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com