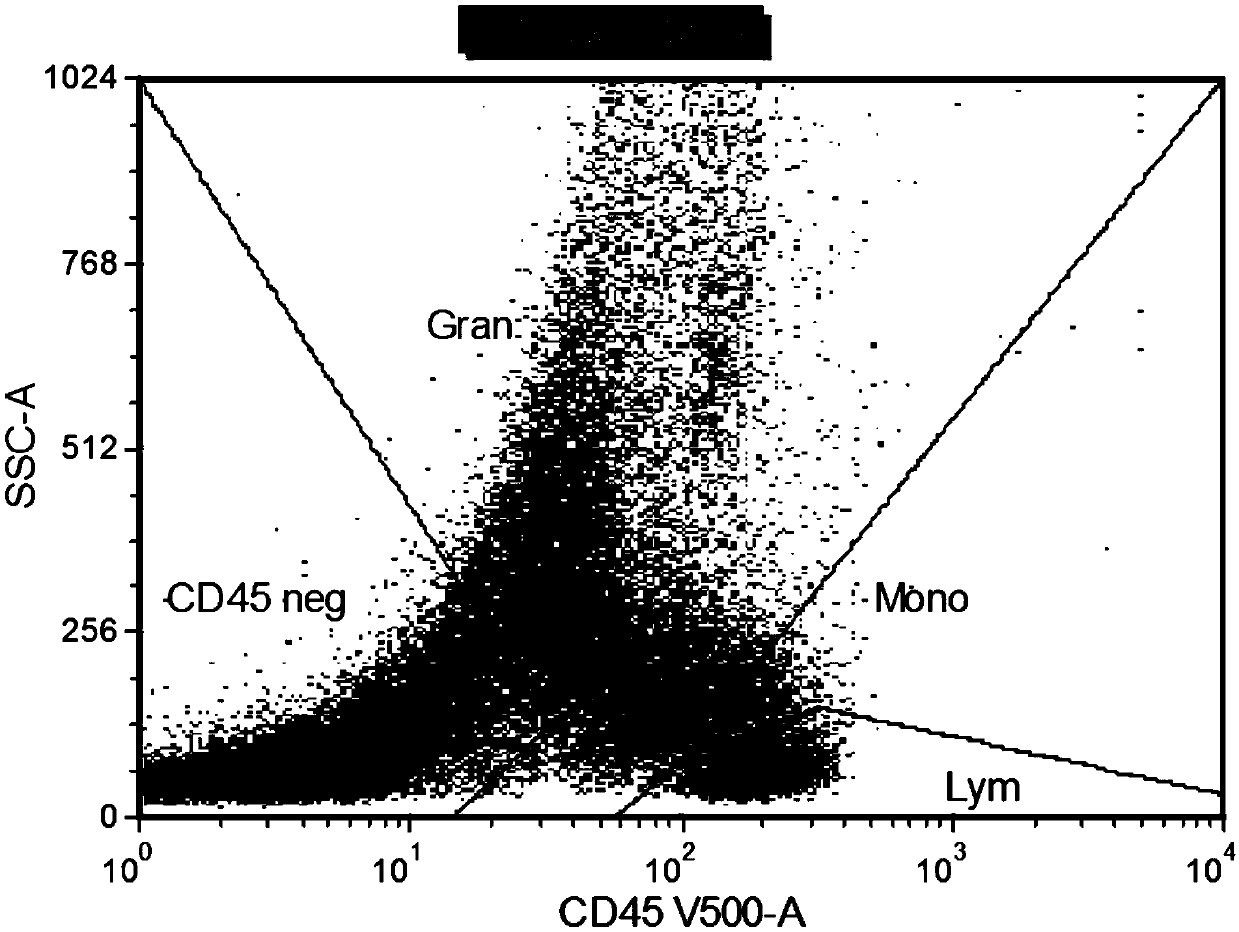
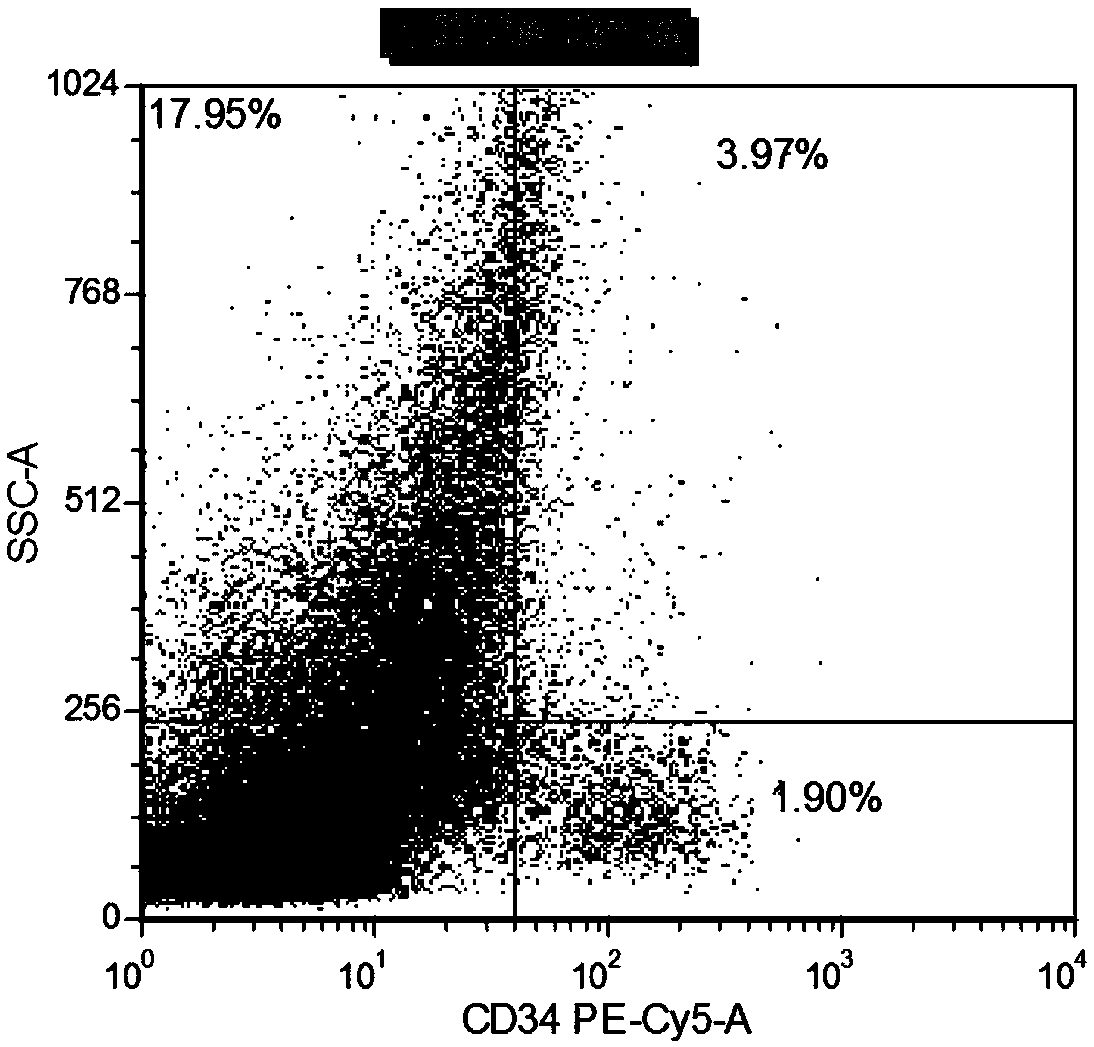
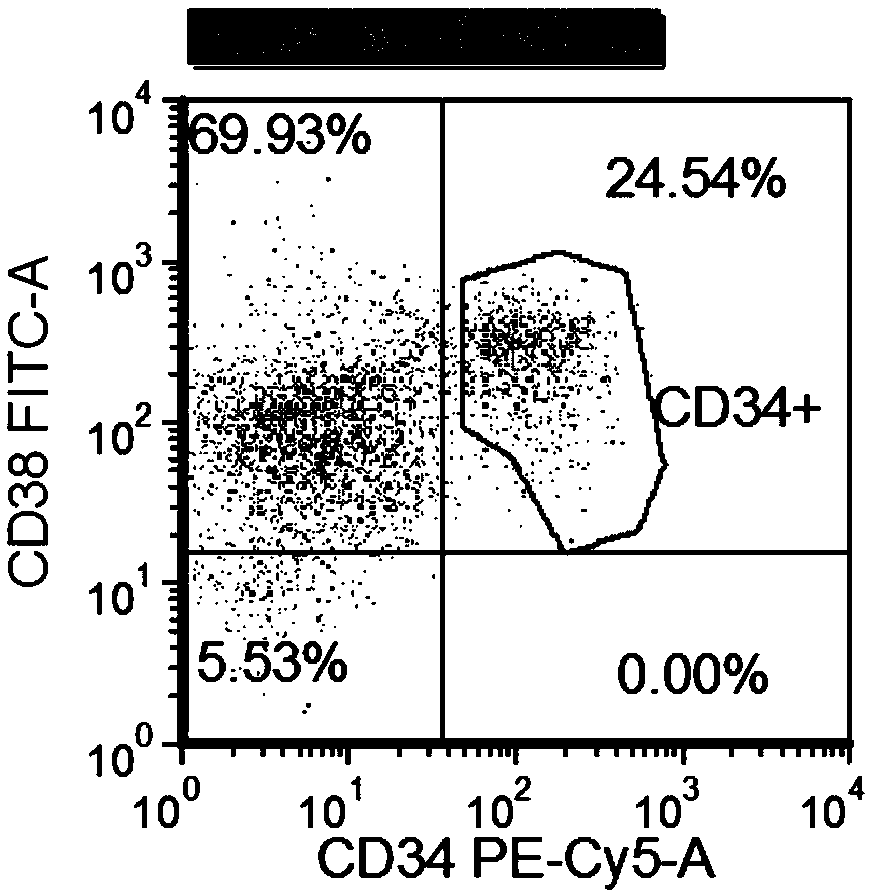
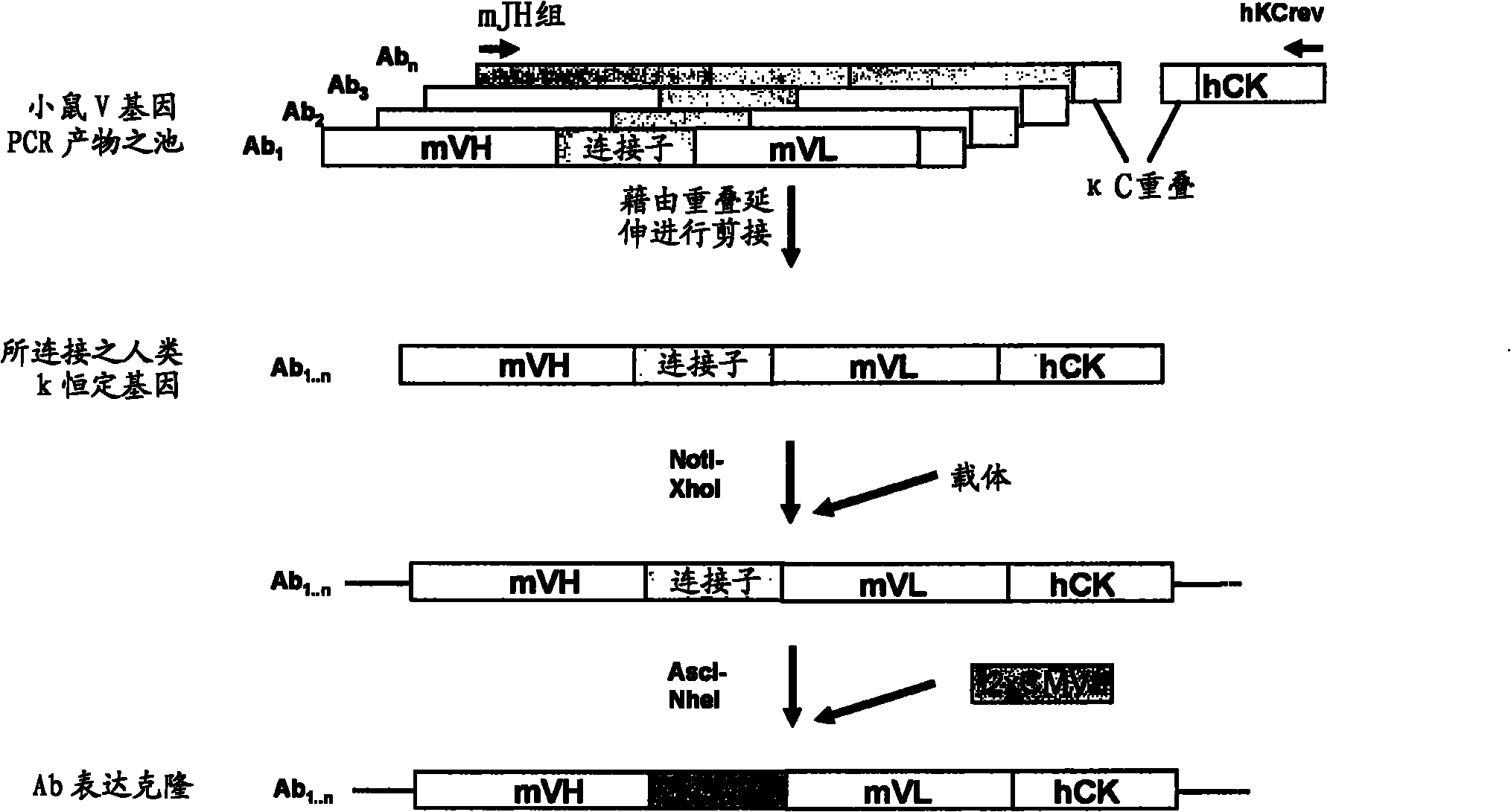
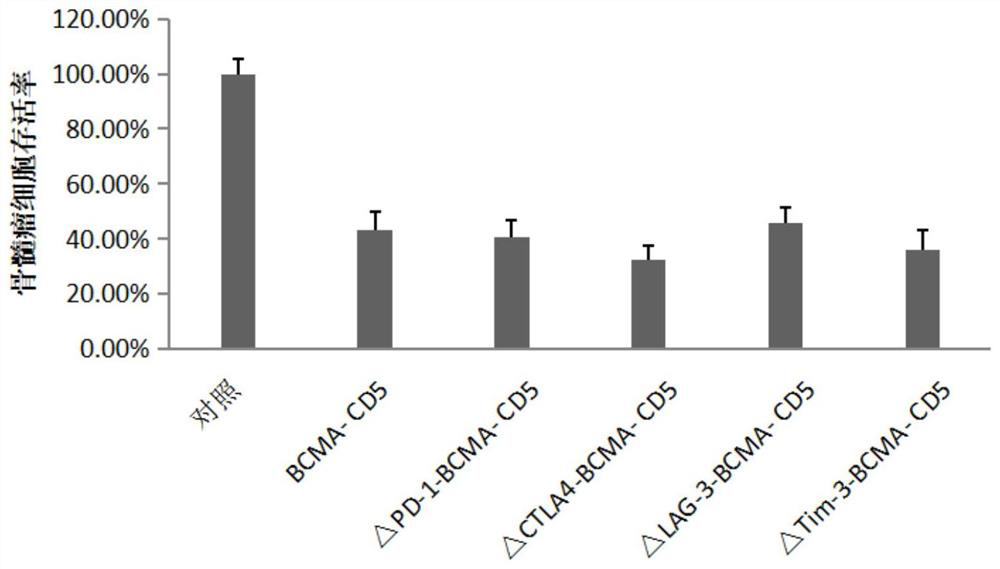
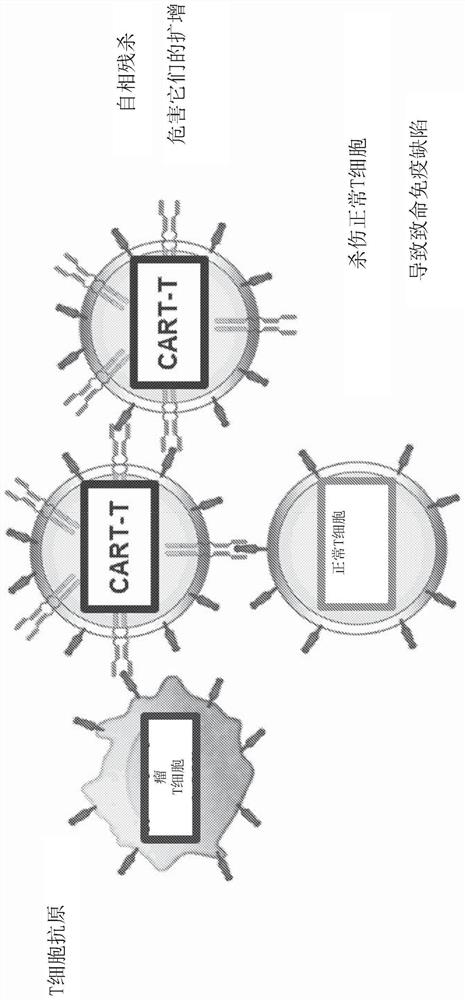
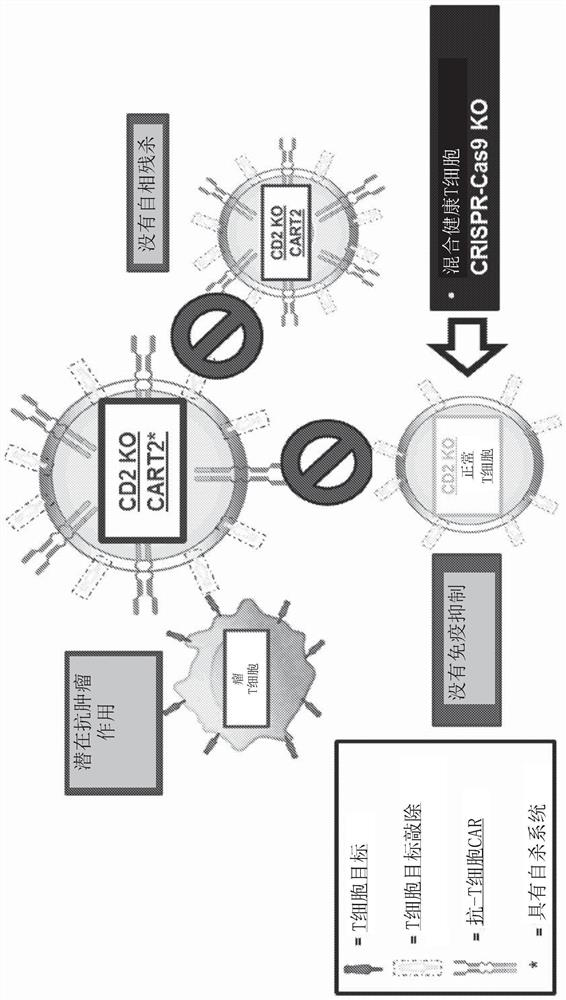
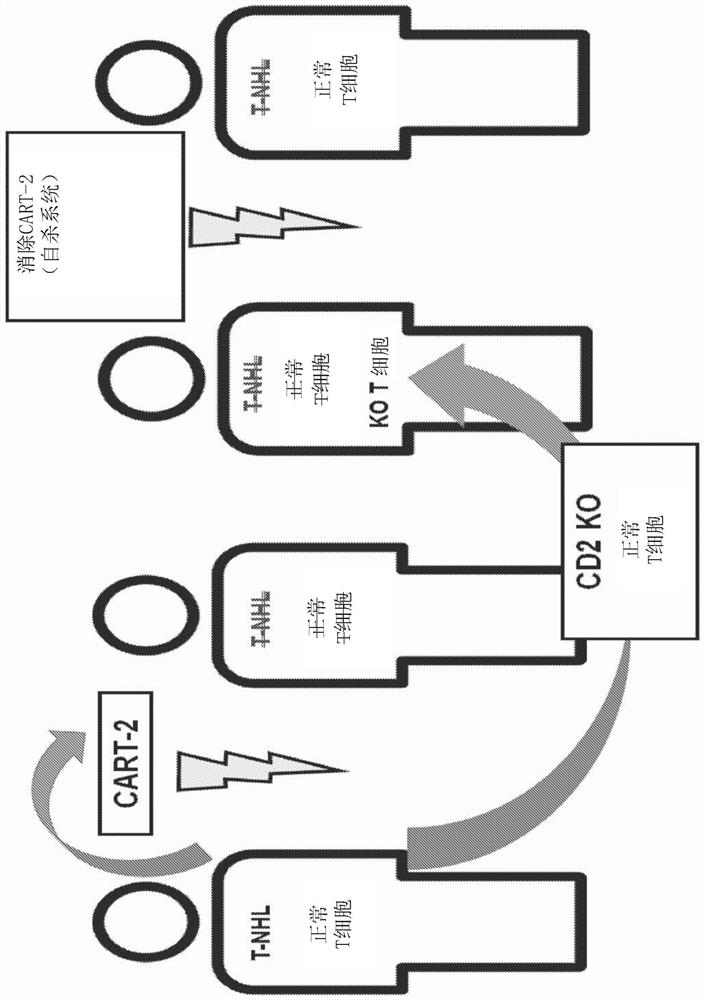
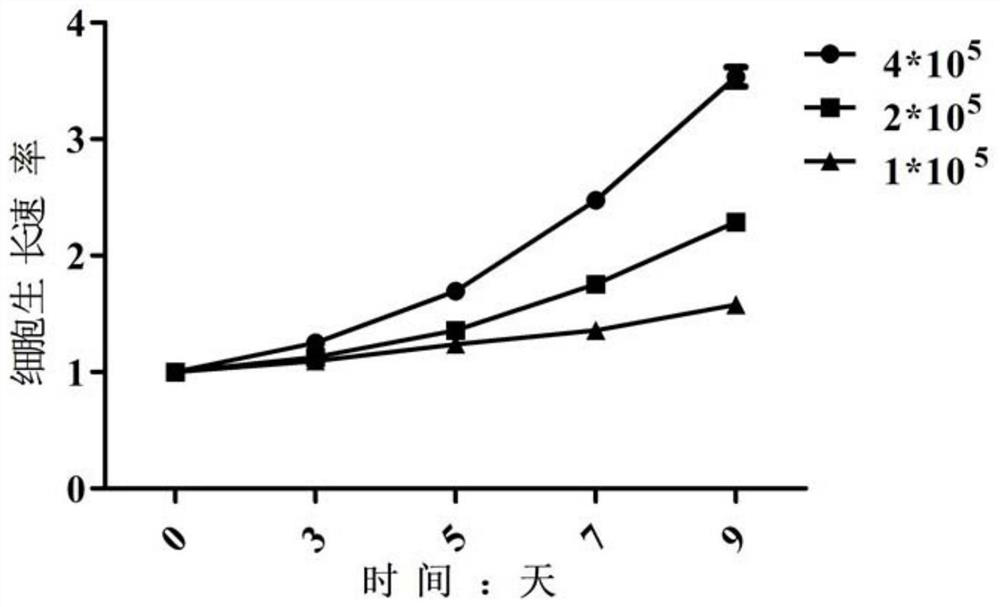
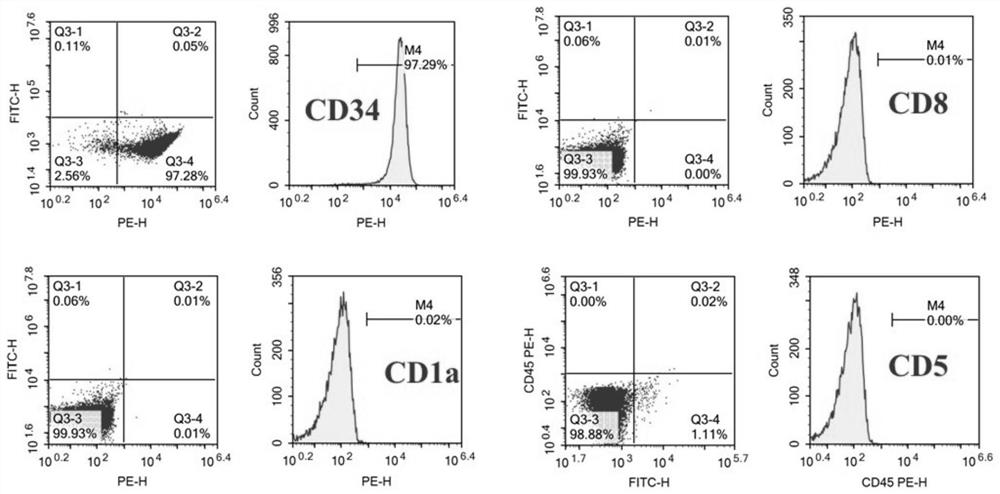
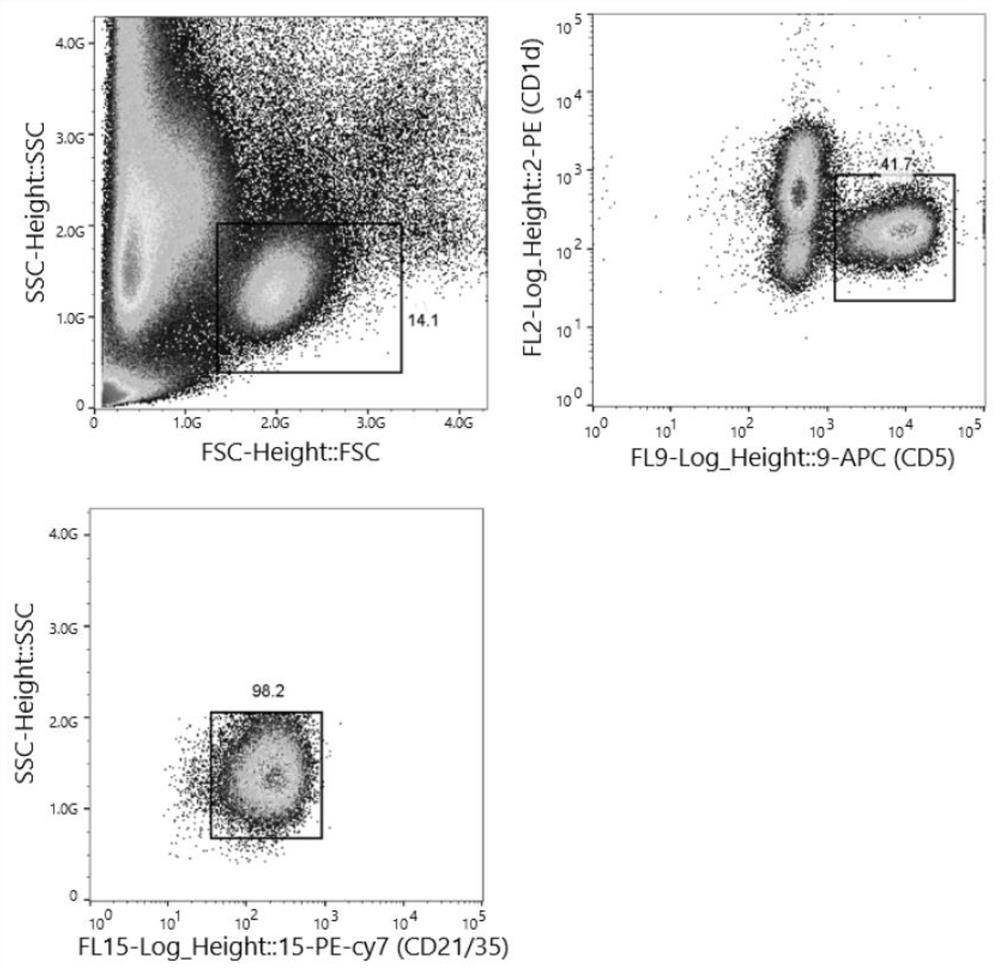
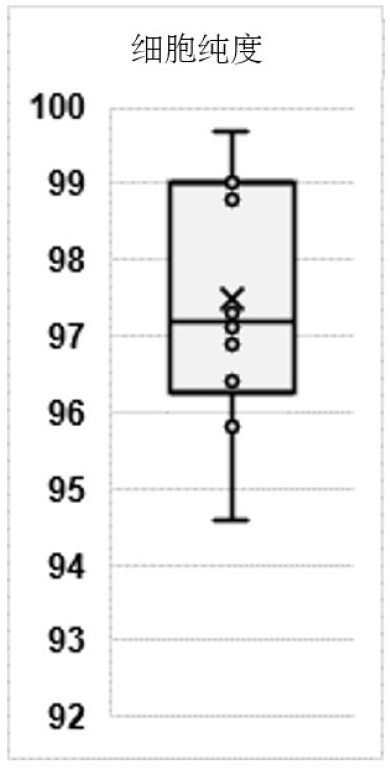
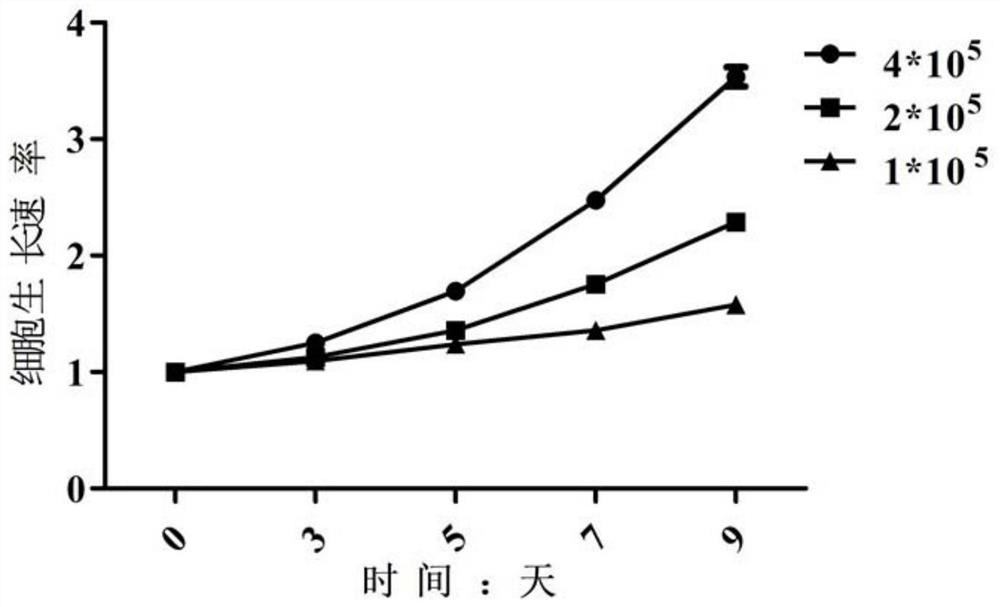
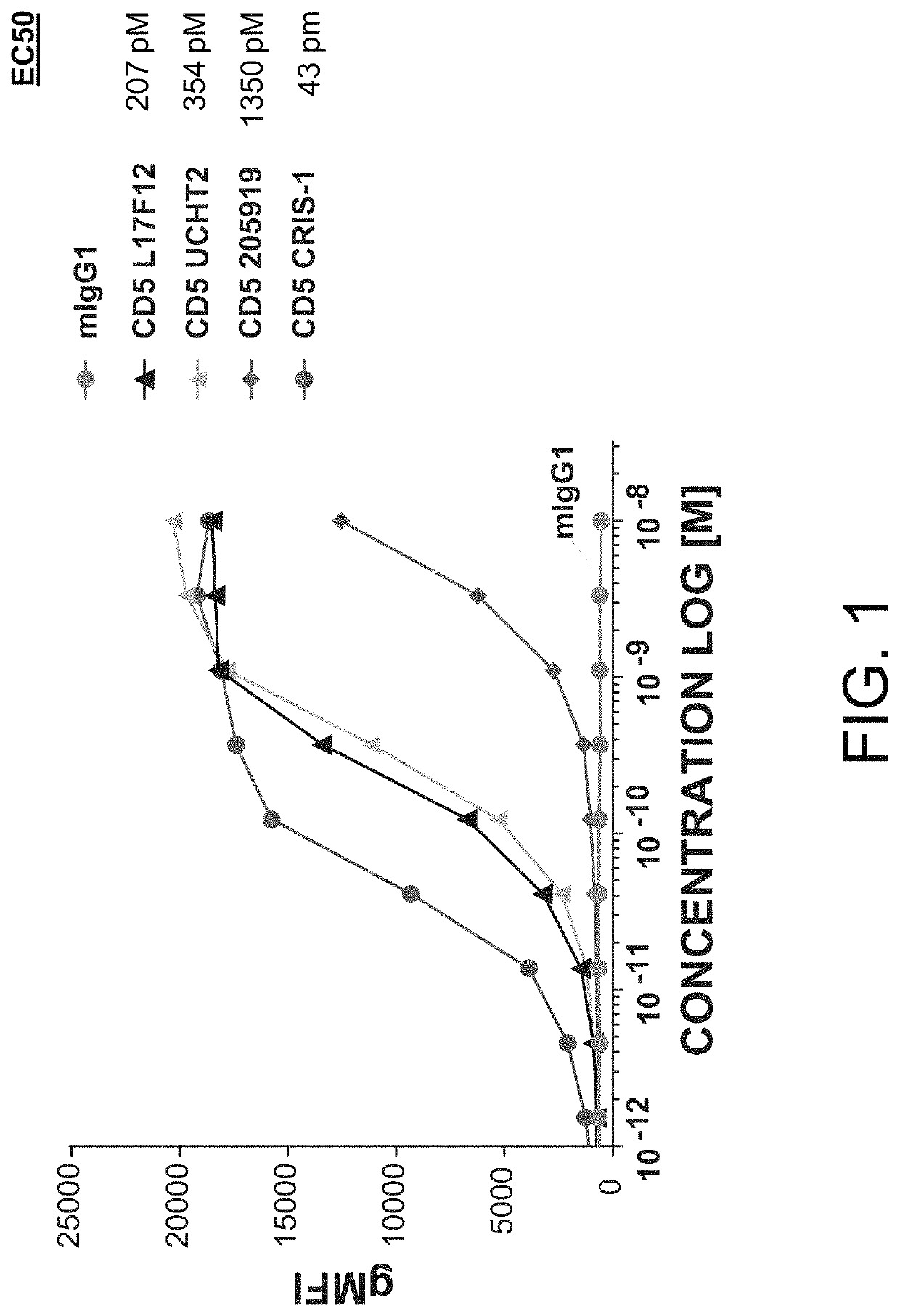
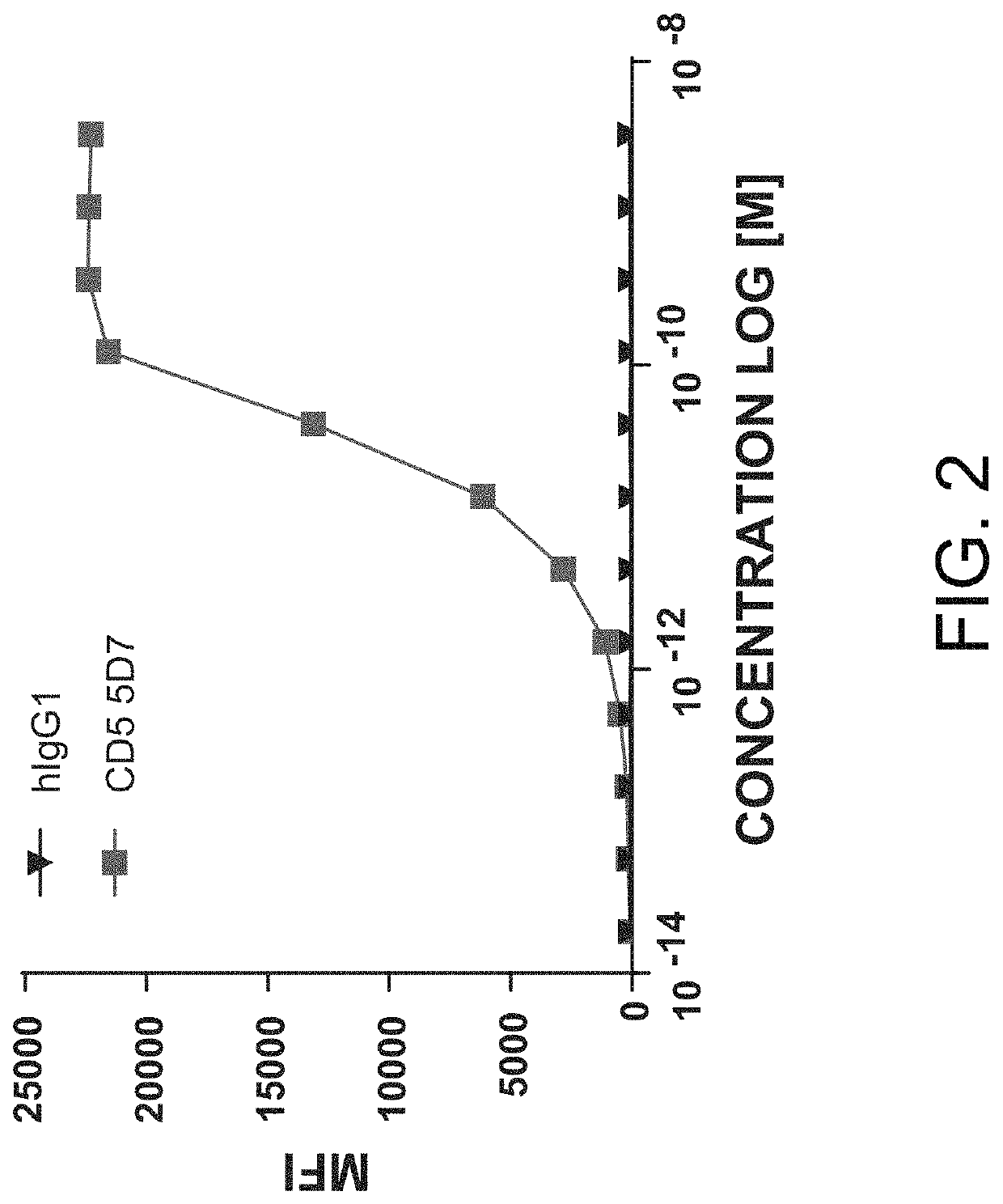
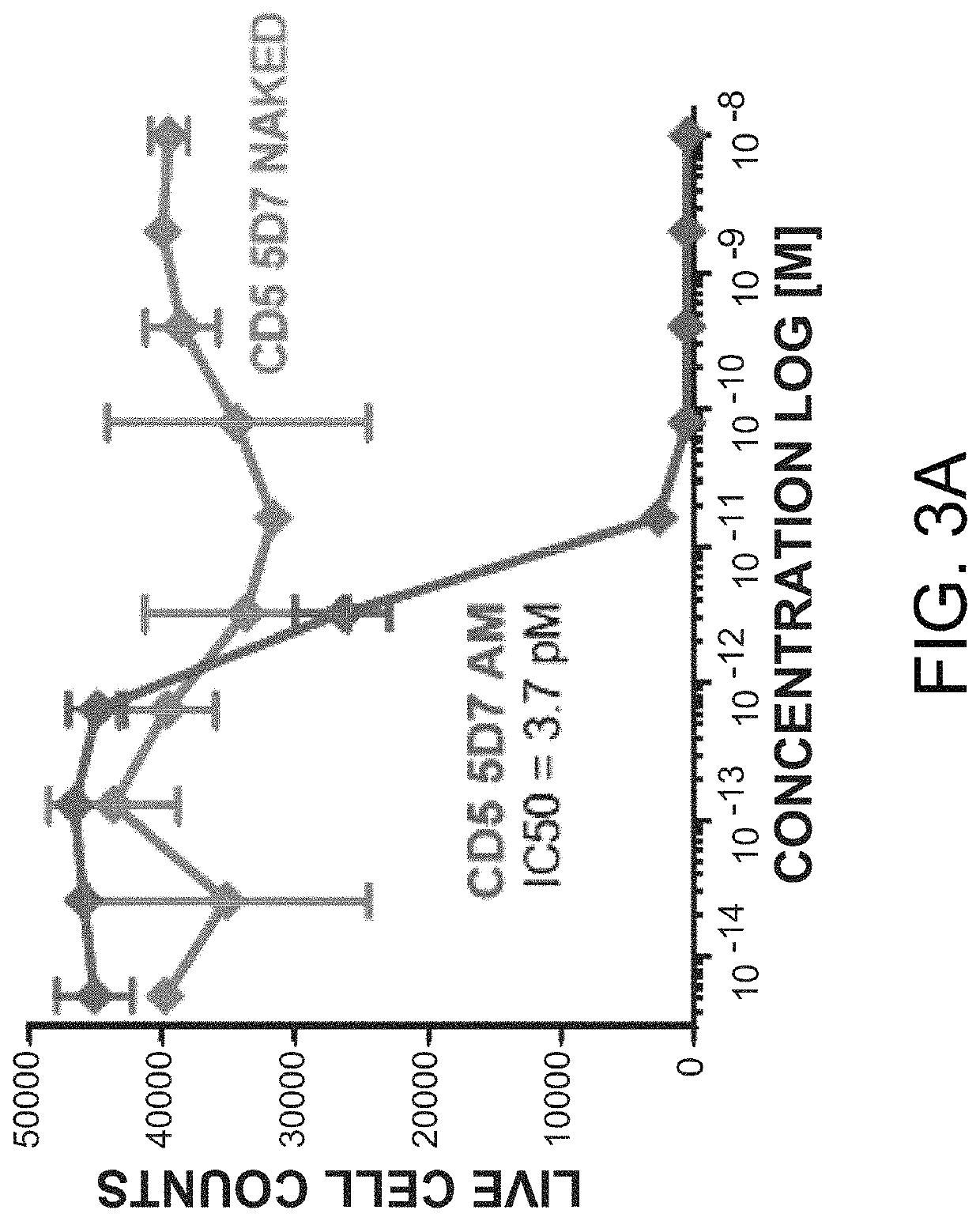
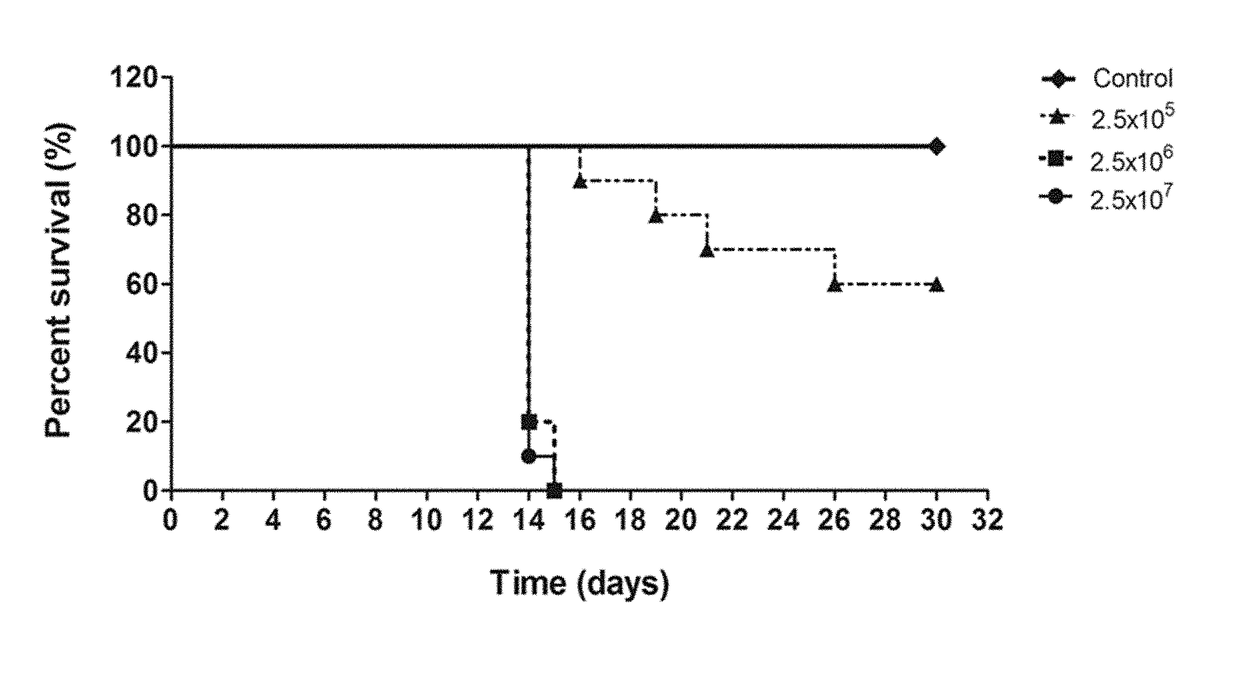
Patents
Literature
31 results about "CD5" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
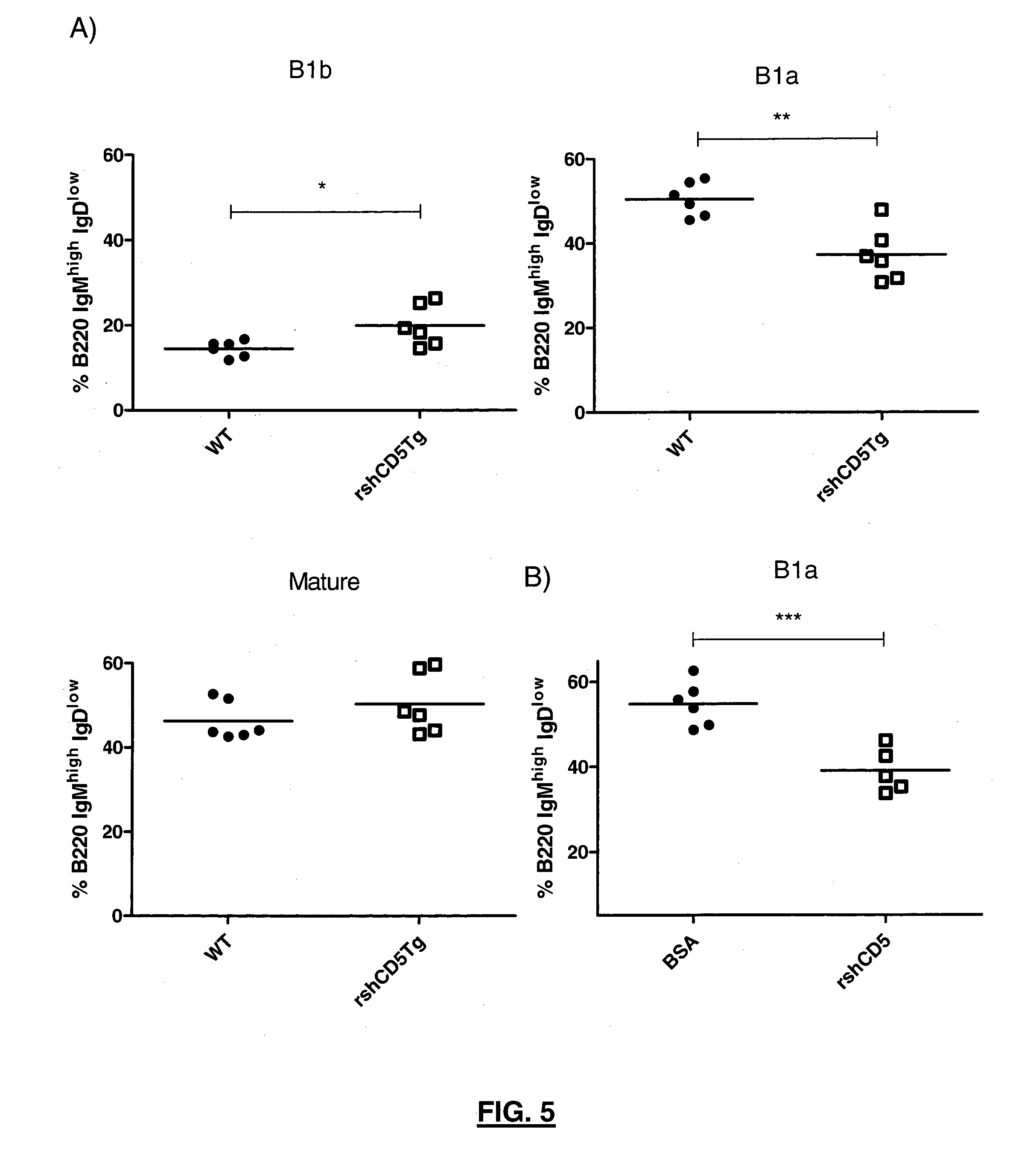
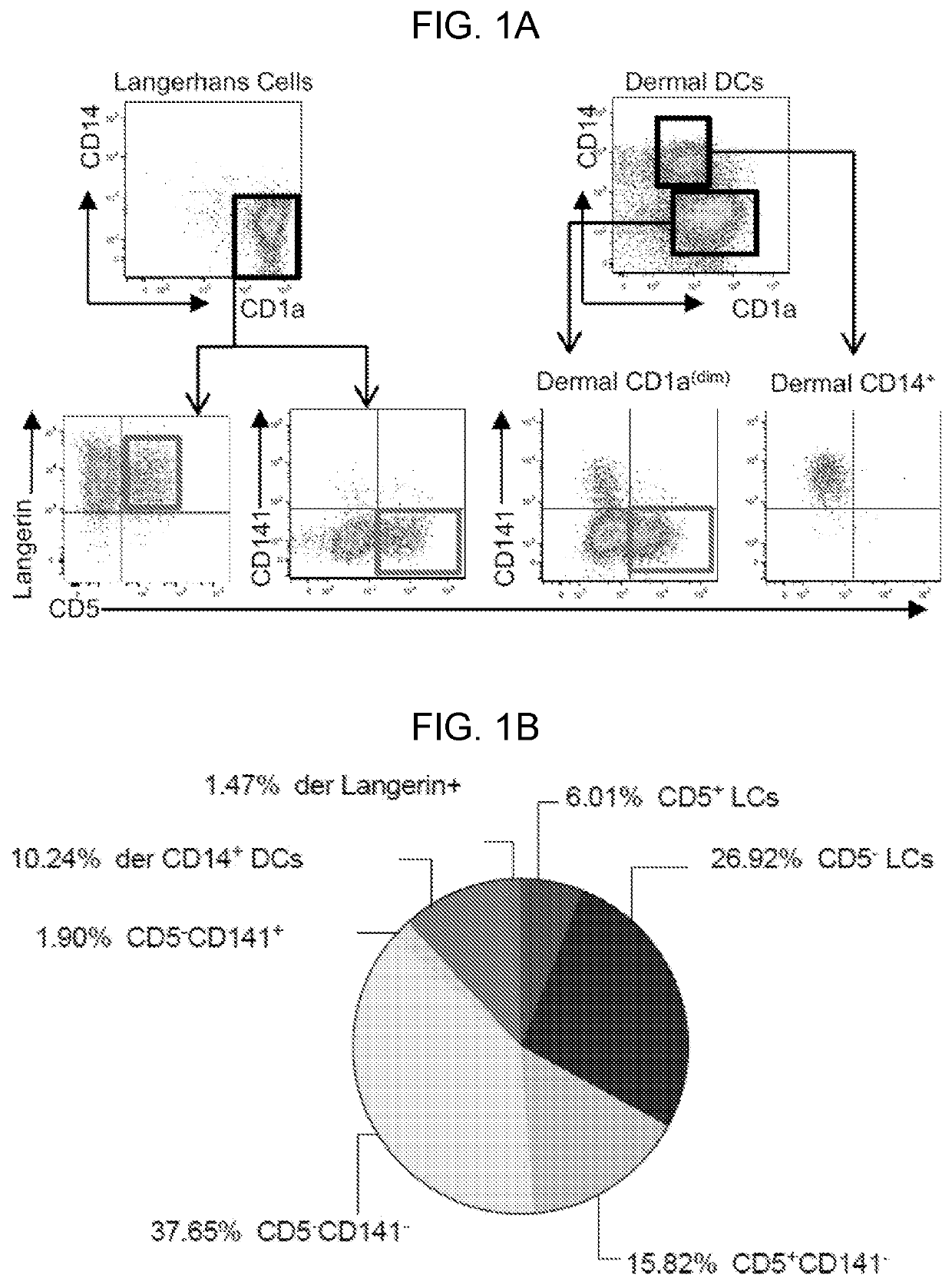
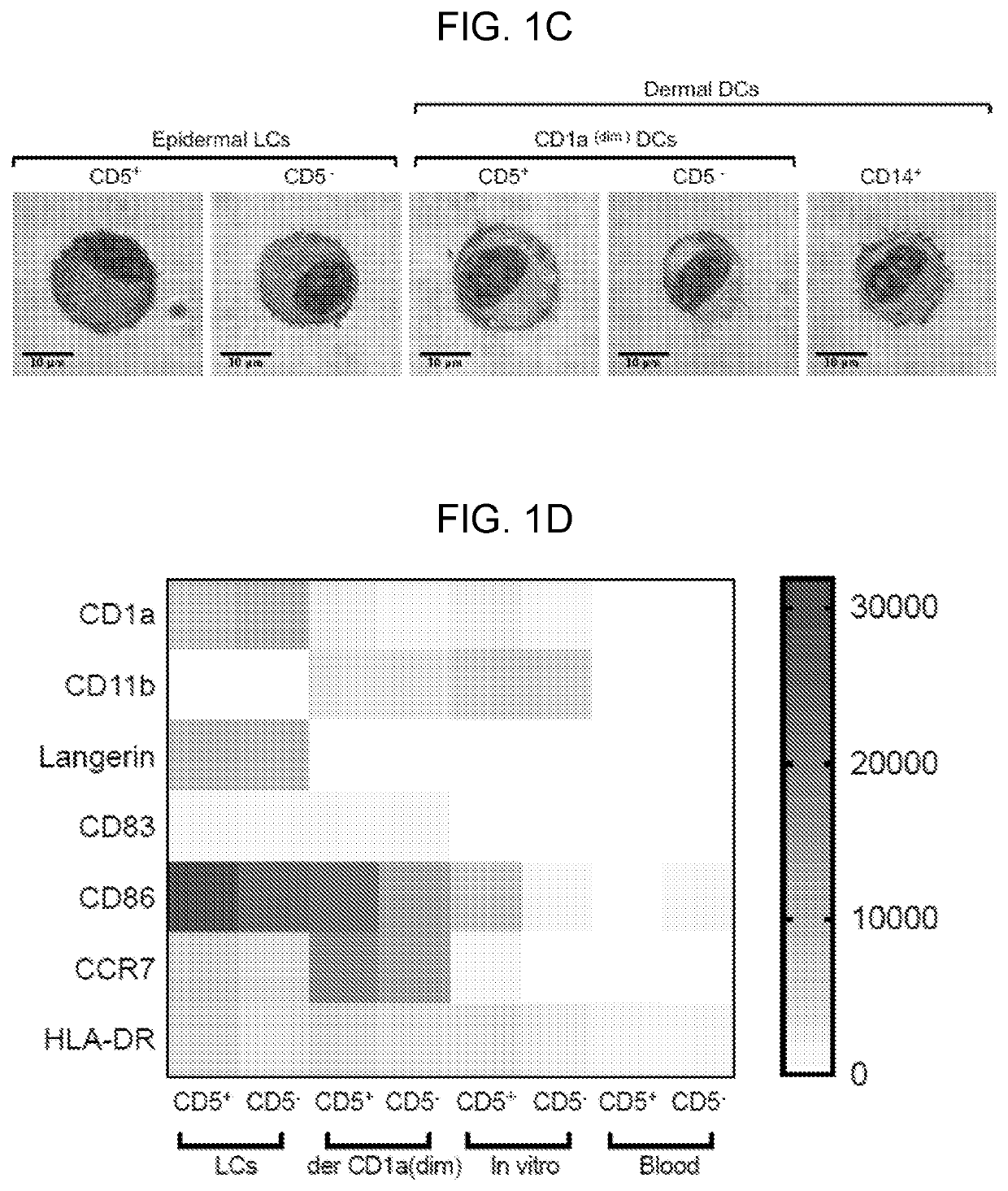
CD5 is a cluster of differentiation expressed on the surface of T cells (various species) and in a subset of murine B cells known as B-1a. The expression of this receptor in human B cells has been a controversial topic and up to date there is no consensus regarding the role of this receptor as a marker of human B cells. B-1 cells have limited diversity of their B-cell receptor due to their lack of the enzyme terminal deoxynucleotidyl transferase (TdT) and are potentially self-reactive. CD5 serves to mitigate activating signals from the BCR so that the B-1 cells can only be activated by very strong stimuli (such as bacterial proteins) and not by normal tissue proteins. CD5 was used as a T-cell marker until monoclonal antibodies against CD3 were developed.
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
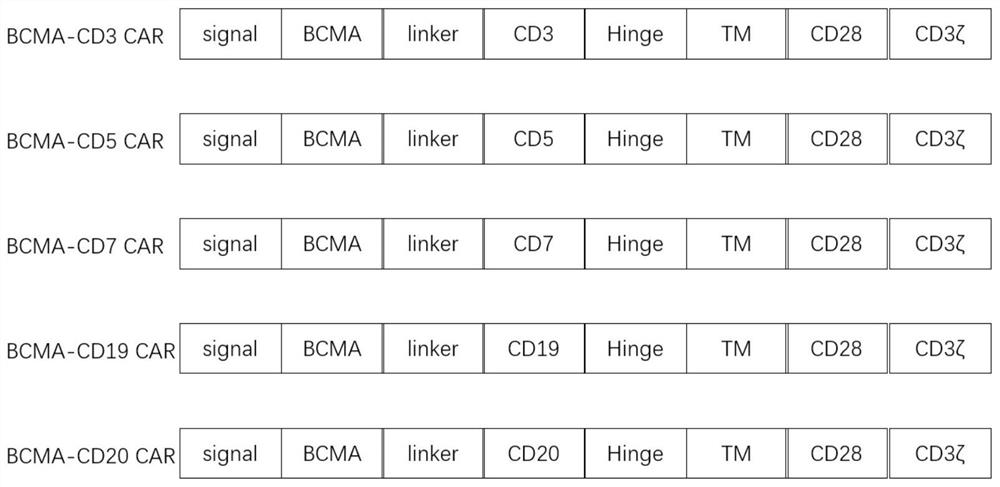
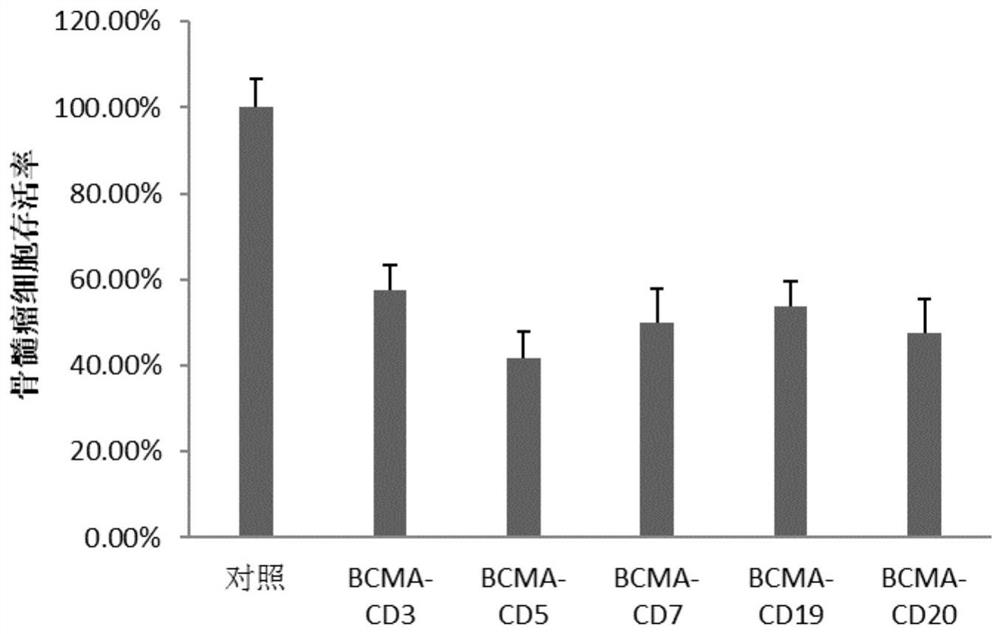
Chimeric antigen receptors (CAR) targeting hematologic malignancies, compositions and methods of use thereof
The present disclosure provides chimeric antigen receptor polypeptides having antigen recognition domains for CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens, and polynucleotides encoding for the same. The present disclosure also provides for engineered cells expressing the polynucleotide or polypeptides. In some embodiments, the disclosure provides methods for treating diseases associated with CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens.
Owner:ICELL GENE THERAPEUTICS LLC +5
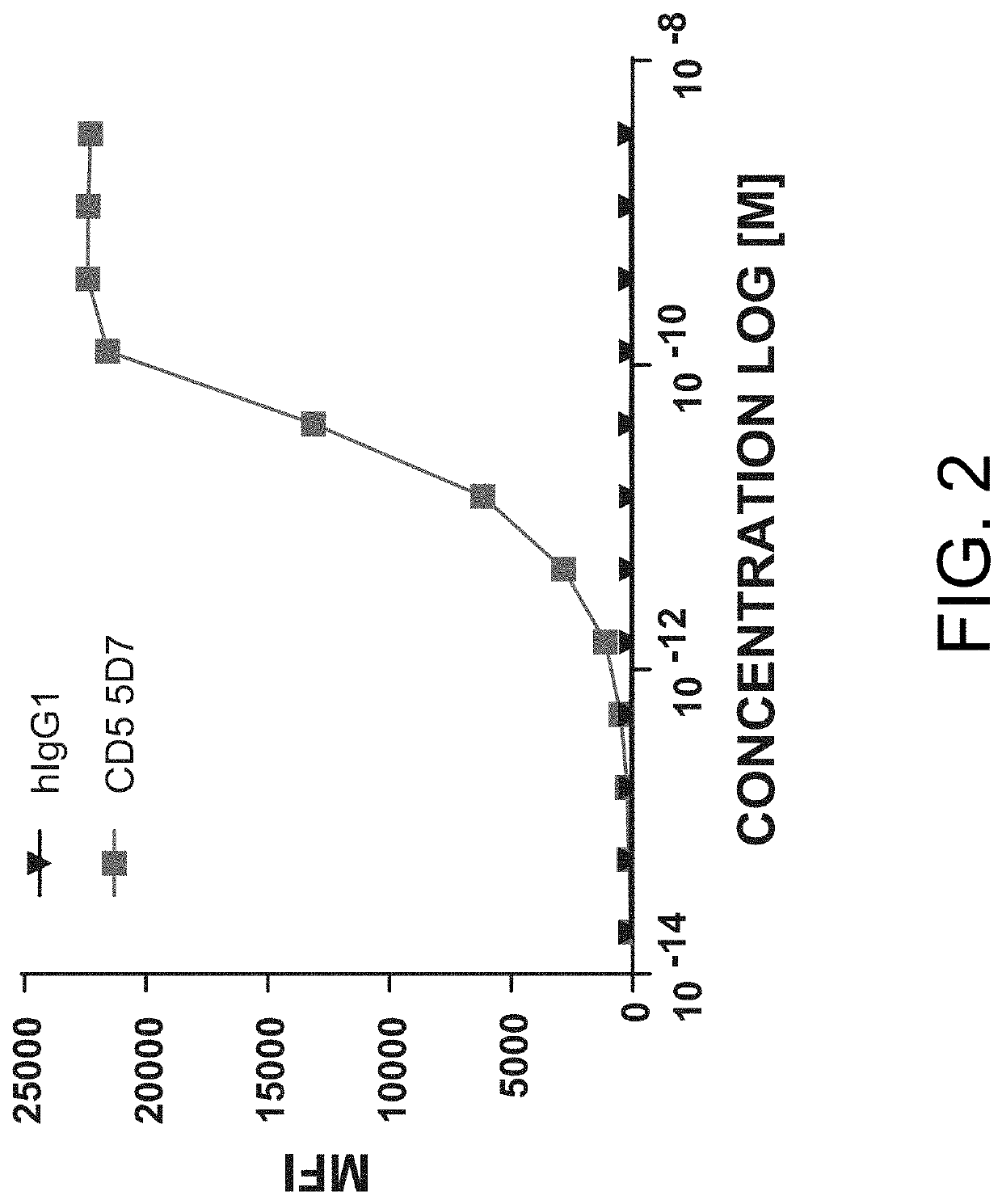
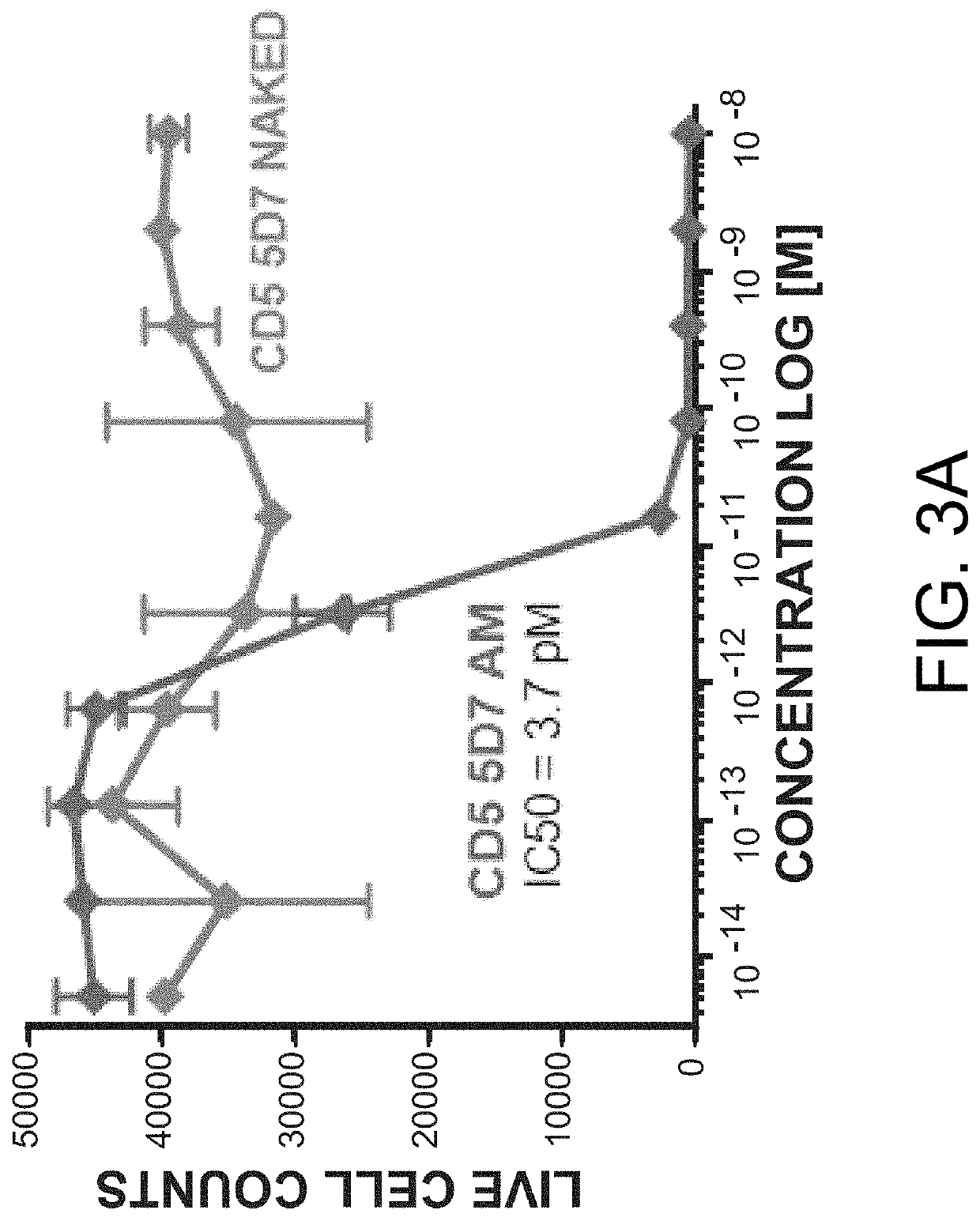
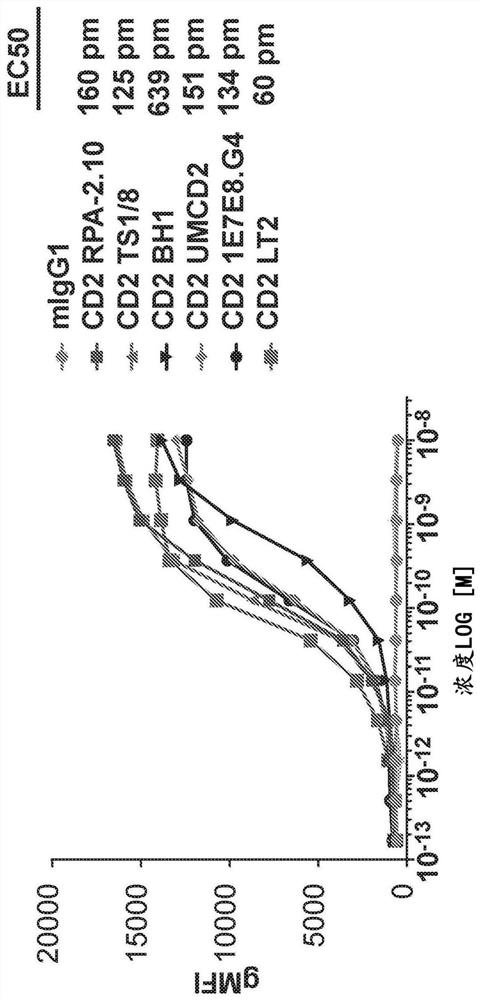
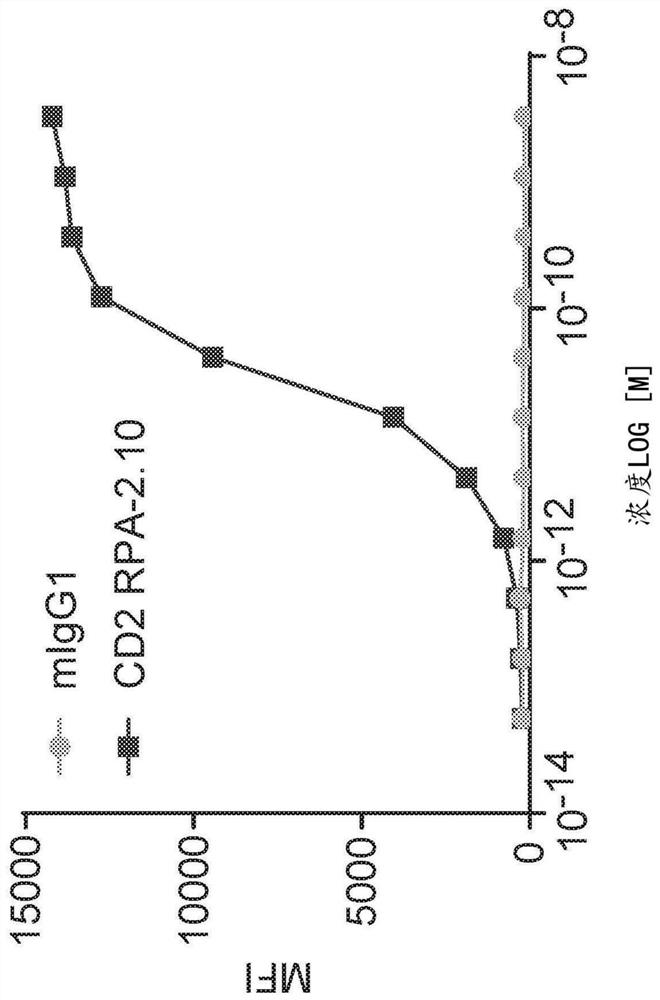
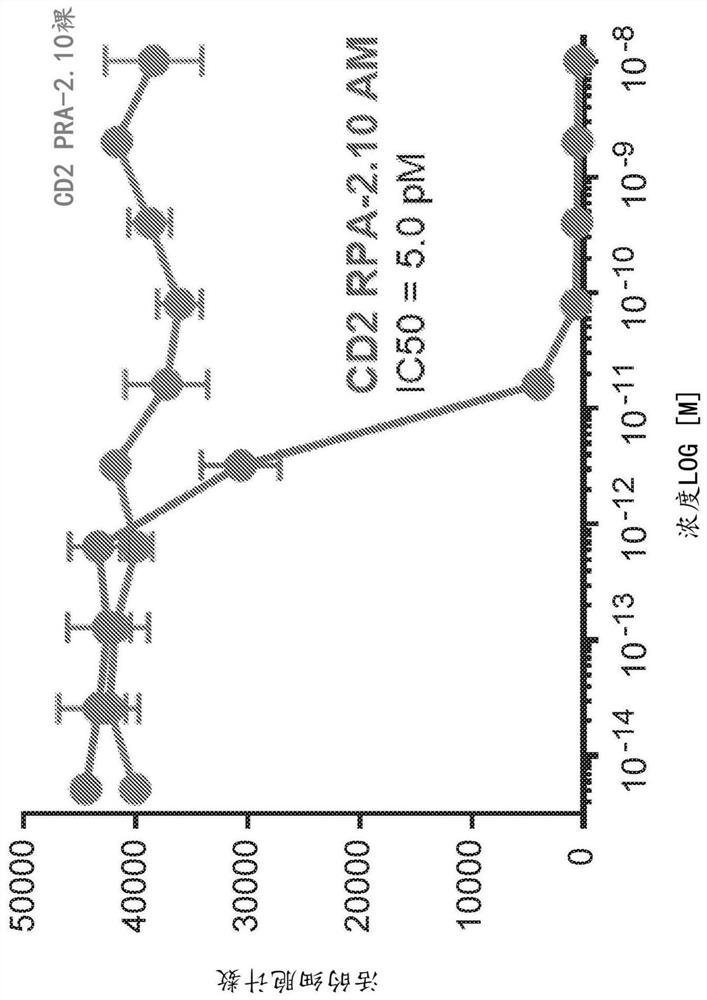
Anti-CD5 antibodies
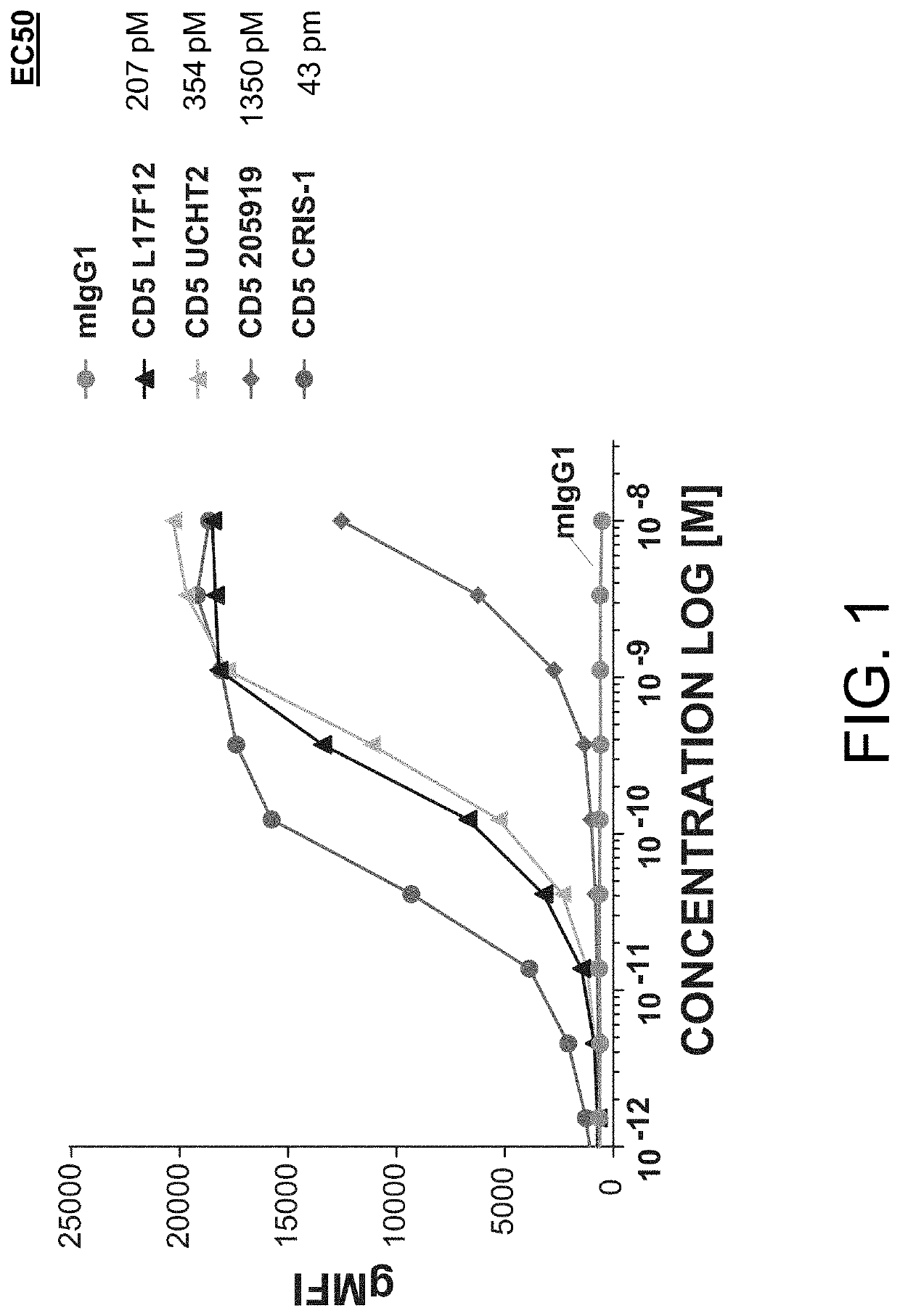
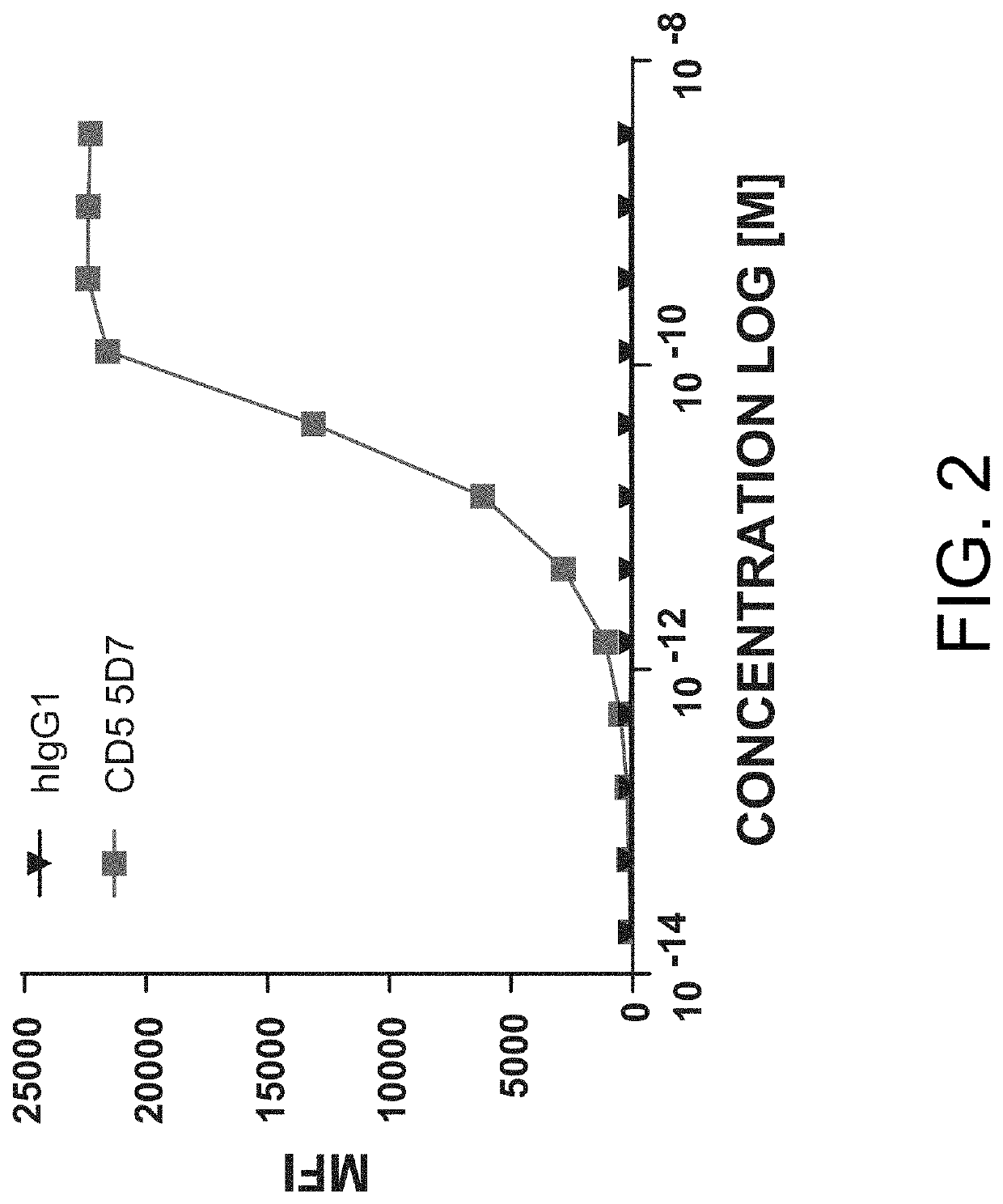
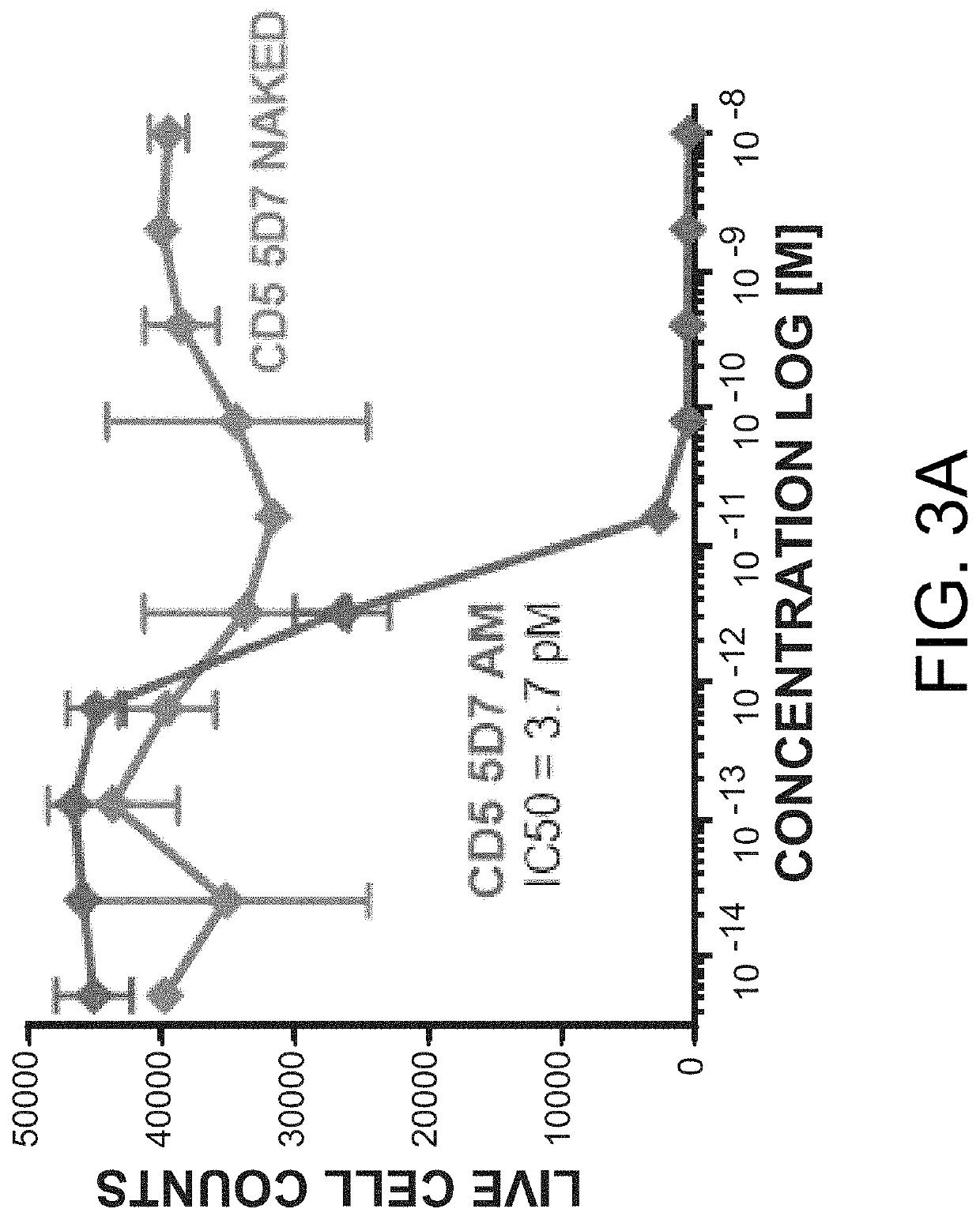
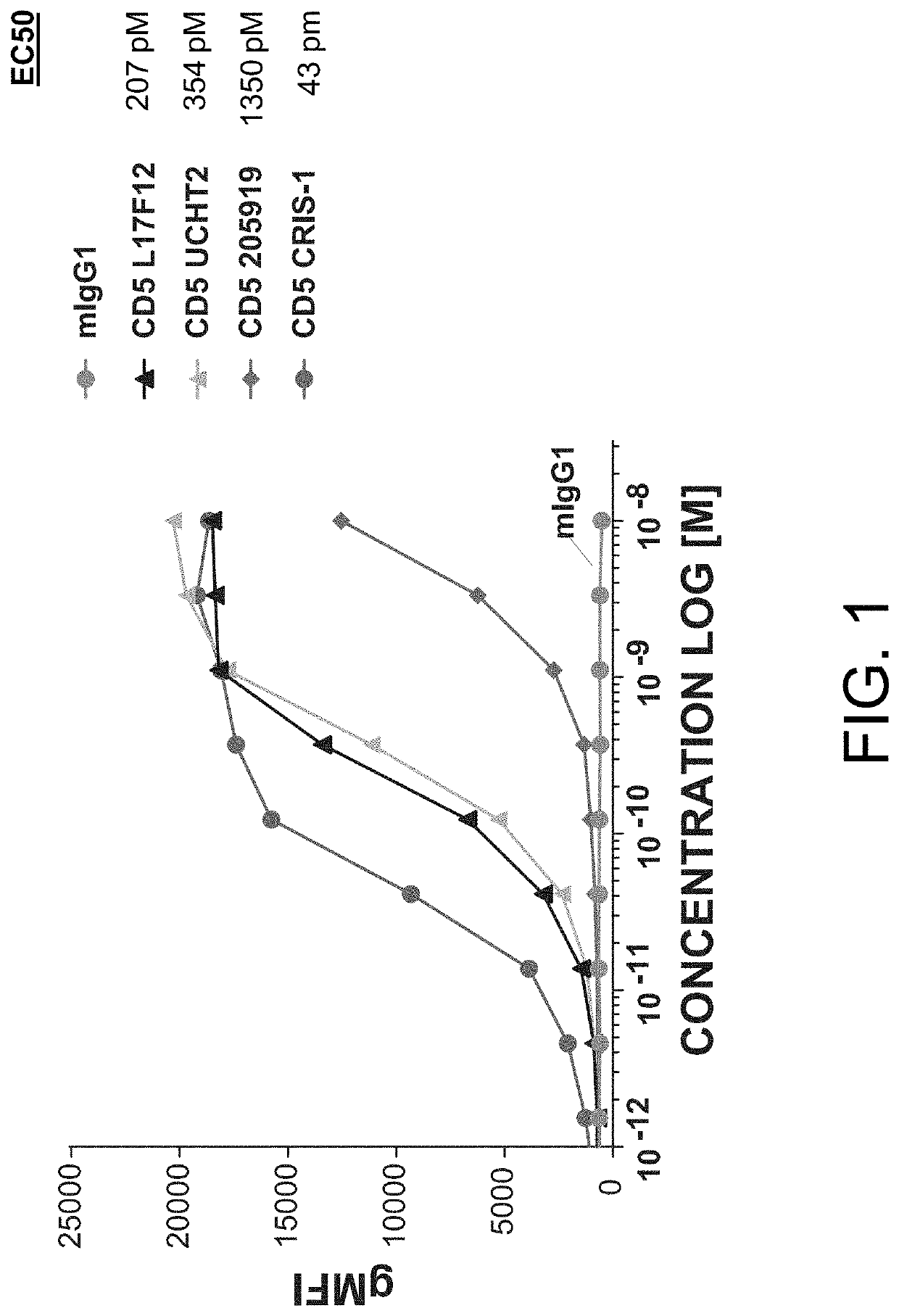
The present invention relates to the field of compositions comprising anti-CD5 antibodies. In particular, the present invention concerns an antibody composition comprising at least two anti-CD5 antibodies capable of binding distinct CD5 epitopes. The invention further concerns bi-specific molecules having the binding specificities of said antibody compositions. The invention also relates topharmaceutical compositions, use of antibody compositions and methods for manufacturing antibody compositions. The invention further relates to cell banks and a method for killing cells.
Owner:SYMPHOGEN AS
Anti-CD5 protein monoclonal antibody as well as cell strain, preparation method and application thereof
ActiveCN113105547AStrong specificityIncreased sensitivityBiological material analysisMicroorganism based processesEscherichia coliCD5
The invention relates to a monoclonal antibody capable of recognizing a human CD5 antigen, a secretory cell strain, a preparation method of the monoclonal antibody and application in immunodetection. According to the technical scheme, amino acids from the 398th site to the 495th site of the C terminal of the CD5 protein are selected as antigen peptides, codon optimization is carried out, a gene segment suitable for being expressed in escherichia coli BL21 is formed, and the finally obtained recombinant protein comprises a CD5 protein segment and a histidine protein tag. A mouse is immunized by the recombinant protein, and the mouse hybridoma cell strain 11H3 capable of efficiently secreting the anti-CD5 protein monoclonal antibody and the anti-CD5 protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing CD5 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Combined reagent and system for evaluating chronic lymphocytic leukemia prognosis
ActiveCN109752548AAccurate identificationEasy to analyzeMaterial analysisIntracellular stainingChronic lymphoblastic leukemia
The invention relates to a combined reagent and a system for evaluating chronic lymphocytic leukemia prognosis, and belongs to the field of medical technology detection. The combined reagent comprisesat least one of the following antibody combinations: an antibody combination 1 including CD5, IgG1, CD3, CD 19 and CD45 antibodies, an antibody combination 2 including CD5, ZAP70, CD3, CD19 and CD45antibodies, and an antibody combination 3 including CD38, CD49d, CD19, CD5 and CD45 antibodies. In the above antibody combinations which aim at CLL (Chronic Lymphocytic Leukemia), the expression of ZAP70, CD38 and CD49d has a great value on an aspect of prognosis evaluation, ZAP70 is accurate, bus since an intracellular dyeing and gating technology restricts clinical application, and the combinedreagent solves the problem to a maximum degree. In addition, two indexes are increased, markers are combined, and chronic lymphocytic leukemia prognosis can be more comprehensively evaluated.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Regulatory b cells and their uses
The present invention relates to a phenotypically distinct CD1dhighCD5+ B cell subset that regulates T cell mediated inflammatory responses through the secretion of interleukin-10 (IL-10). The invention also relates to the use of these IL-10 producing regulatory B cells in the manipulation of immune and inflammatory responses, and in the treatment of disease. Therapeutic approaches involving adoptive transfer of these regulatory B cells, or expansion of their endogenous levels for controlling autoimmune or inflammatory diseases and conditions are described. Ablation of this subset of regulatory B cells, or inhibition of their IL-10 production can be used to upregulate immunodeficient conditions, and / or to treat tumors / cancer. Diagnostic applications also are encompassed.
Owner:DUKE UNIV
Soluble protein cd5 or cd6 for the treatment of cancer or tumor or for use as an adjuvant
InactiveUS20140215644A1Organic active ingredientsBacterial antigen ingredientsImmunologic disordersAutoimmune condition
The present invention discloses soluble isoforms of CD5 and CD6, which are scavenger-like lymphocyte receptors, for use in the prophylaxis or therapy of disorders or in therapeutic interventions, which would benefit from an exacerbated immune response to either endogenous or exogenous antigens, resulting from a decrease in lymphocyte subpopulations with regulatory functions and / or increase in lymphocyte subpopulations with effector functions. Special disorders are cell growth disorders, and chronic infections of bacterial, viral, fungal or parasitic origin. The invention also provides animal models for the study and the prophylaxis / treatment of autoimmune diseases, cancer, and chronic infections.
Owner:FUNDACIO CLINIC PER A LA RECERCA BIOMEDICA +2
Oligopeptide molecules, and preparation methods and applications thereof
InactiveCN104817617ASmall molecular weightEasy to synthesizeDipeptide ingredientsAntipyreticWound healingTreatment effect
Disclosed are oligopeptide molecules, preparation methods therefor and uses thereof. Said oligopeptides comprise CD1、CD2、CD3、CD4、CD5、CD6、CD7、CD8、CD9、CD10、CD11、CD12、CD13 and CD14. The amino acid sequences are shown in SEQ ID NO: 1-14 respectively. The oligopeptides can be prepared by synthesis, extraction or genetic engineering etc. Also disclosed are the uses of said oligopeptides in the preparation of anti-inflammatory and analgesic, wound healing and immunity enhancing drugs.
Owner:陈光健
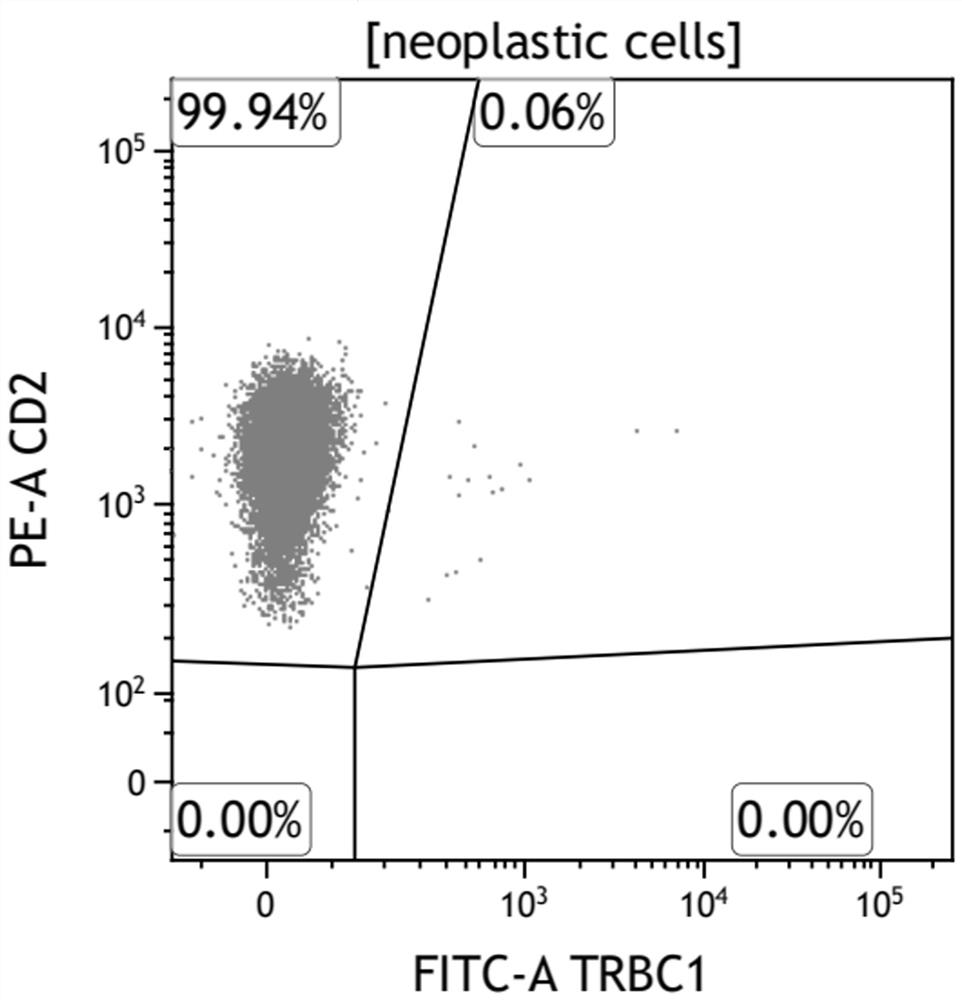
Kit for rapidly detecting peripheral T cell lymphoma and use method thereof
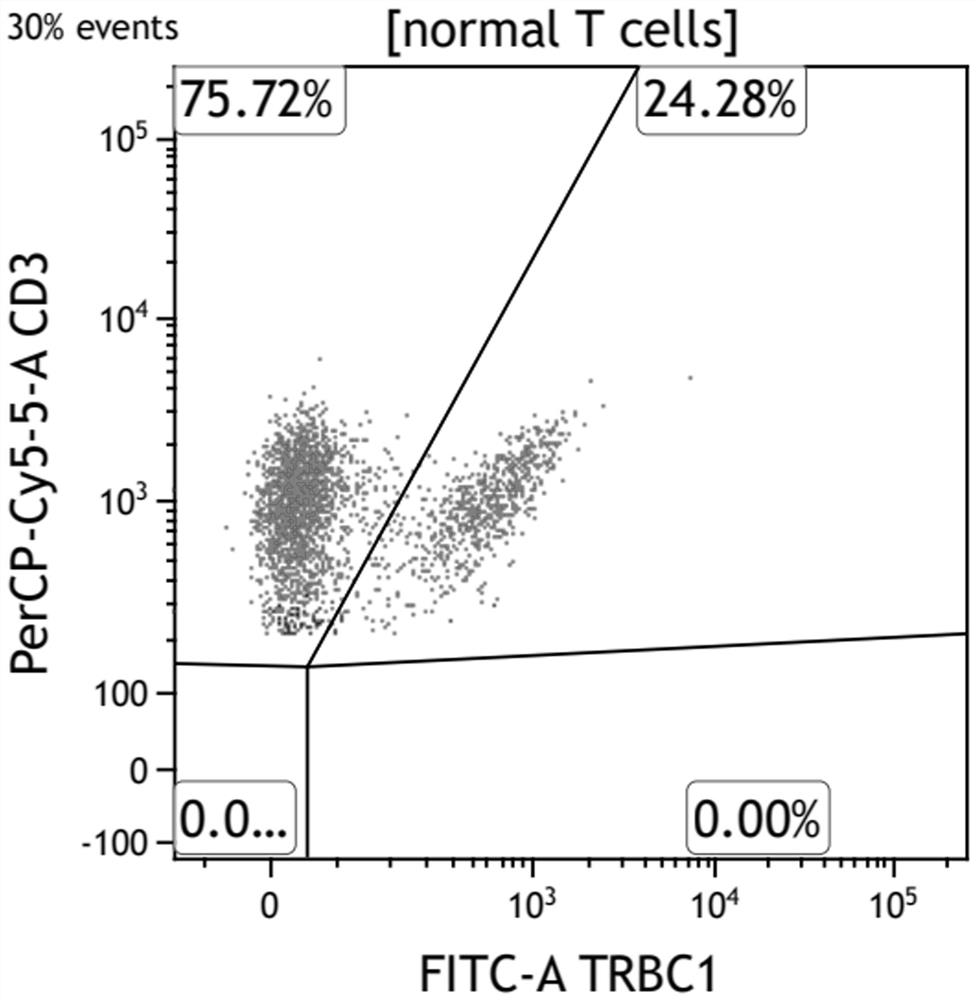
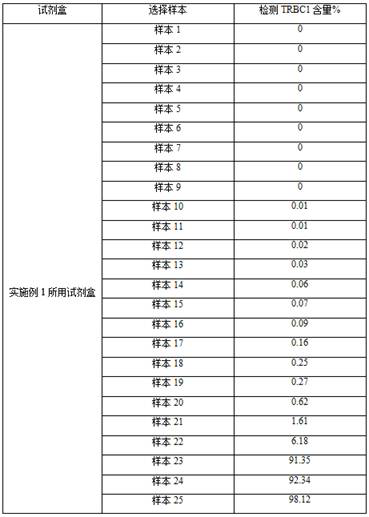
The invention belongs to the field of immunological detection, and relates to a kit, in particular to a kit for rapidly detecting peripheral T cell lymphoma and a use method of the kit. The kit comprises a reagent I and a reagent II, the reagent I comprises two groups of monoclonal antibody mixtures, and the first group of monoclonal antibody mixture comprises TRBC1, CD2, CD3, CD5, CD7, CD16, CD56, CD57 and CD45; the second group of monoclonal antibody mixture comprises the following components: TRBC1, TCR-gamma delta, CD3, CD8, CD4, CD7 and CD45; and the reagent II is hemolysin. The kit provided by the invention realizes the rapid and accurate detection of the peripheral T cell lymphoma (PTCL), is simple and convenient, greatly reduces the requirement on the sample size, and reduces the burden of a patient.
Owner:河南凯普瑞生物技术有限公司
CAR-NK cell as well as preparation method and application thereof
PendingCN114605560AIncrease lethalityDisarm immune escapePolypeptide with localisation/targeting motifImmunoglobulin superfamilyTumor targetCD5
Owner:JIANGSU MOBILI BIOTECH CO LTD
Use of cd2/5/7 knock-out Anti-cd2/5/7 chimeric antigen receptor t cells against t cell lymphomas and leukemias
The present invention includes compositions and methods for treating T cell lymphomas and leukemias. In certain aspects, the compositions and methods include CAR T cells targeting CD2, CD5, or CD7 and modified cells, wherein CD2, CD5, or CD7 has been knocked-out.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Use of CD2/5/7 Knock-Out Anti-CD2/5/7 Chimeric Antigen Receptor T cells Against T Cell Lymphomas and Leukemias
ActiveUS20220073639A1Unlikely can be integratedAnimal cellsPeptide/protein ingredientsAntigen receptorCD5
The present invention includes compositions and methods for treating T cell lymphomas and leukemias. In certain aspects, the compositions and methods include CAR T cells targeting CD2, CD5, or CD7 and modified cells wherein CD2, CD5, or CD7 has been knocked-out.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Chimeric antigen receptors (CARS) targeting hematologic malignancies, compositions and methods of use thereof
The present disclosure provides chimeric antigen receptor polypeptides having antigen recognition domains for CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens, and polynucleotides encoding for the same. The present disclosure also provides for engineered cells expressing the polynucleotide or polypeptides. In some embodiments, the disclosure provides methods for treating diseases associated with CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens.
Owner:ICELL GENE THERAPEUTICS LLC
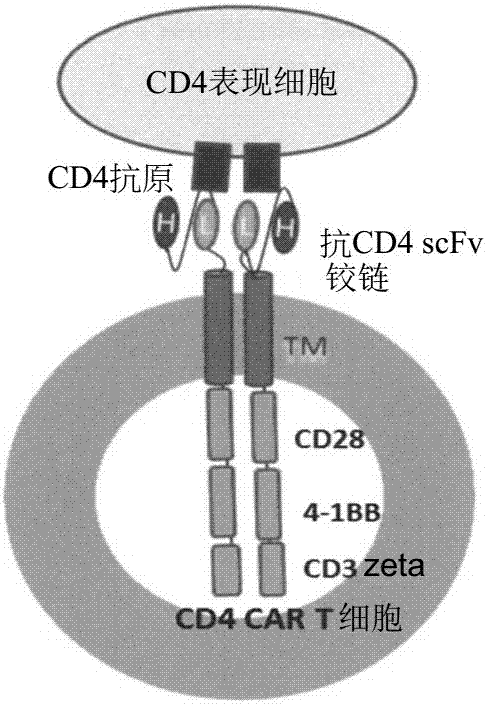
CD5 chimeric antigen receptor for adoptive T cell therapy
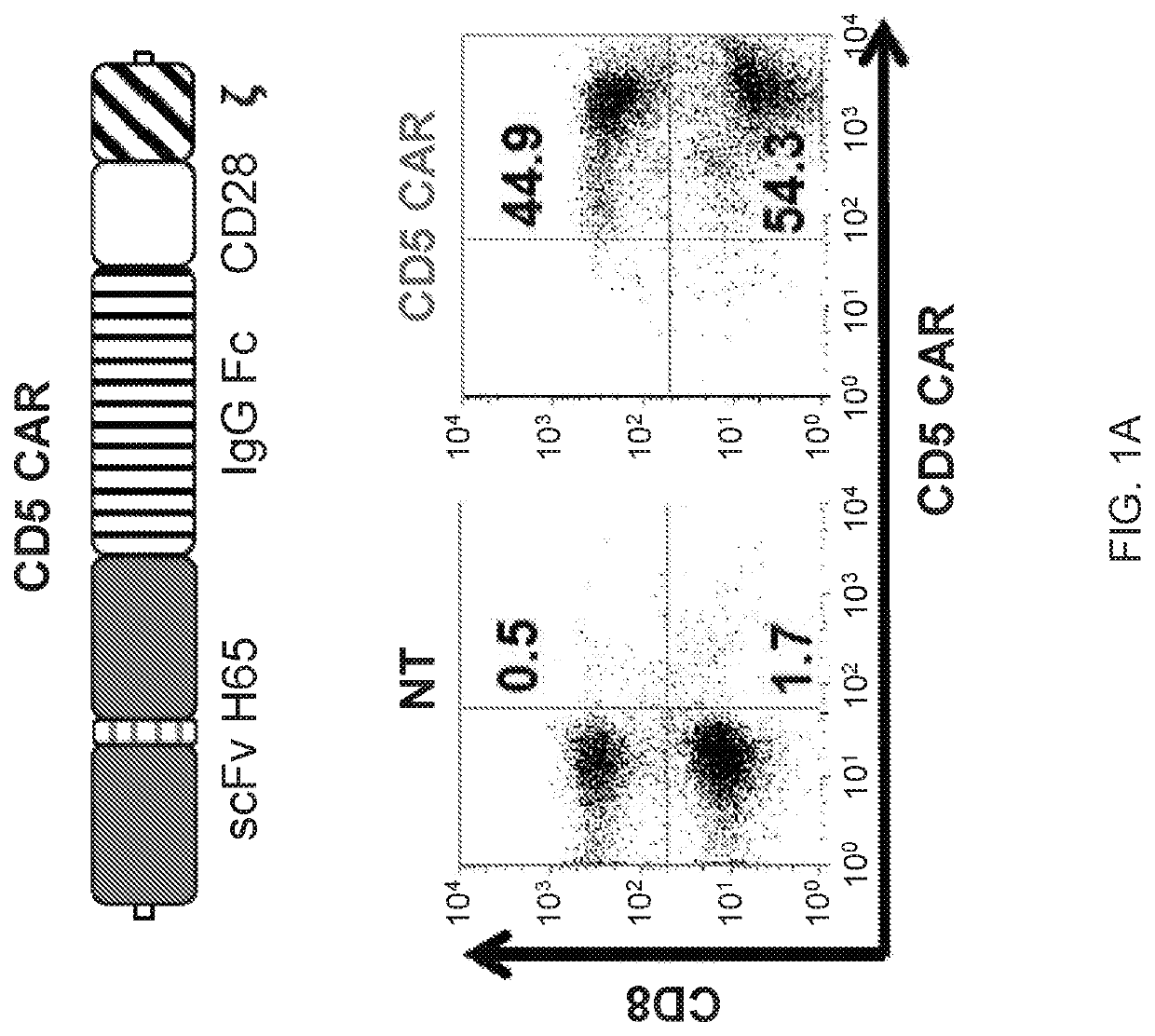
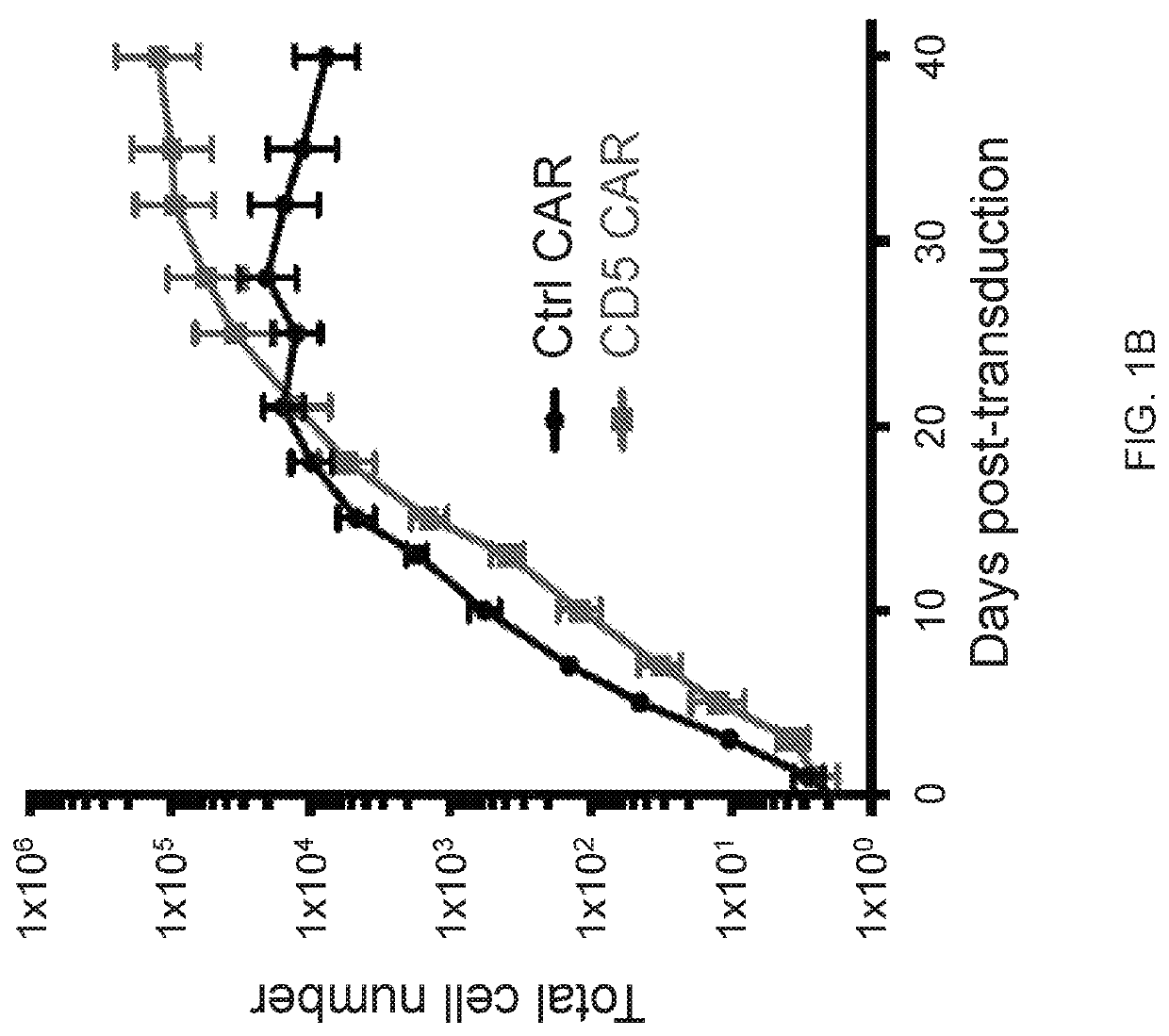
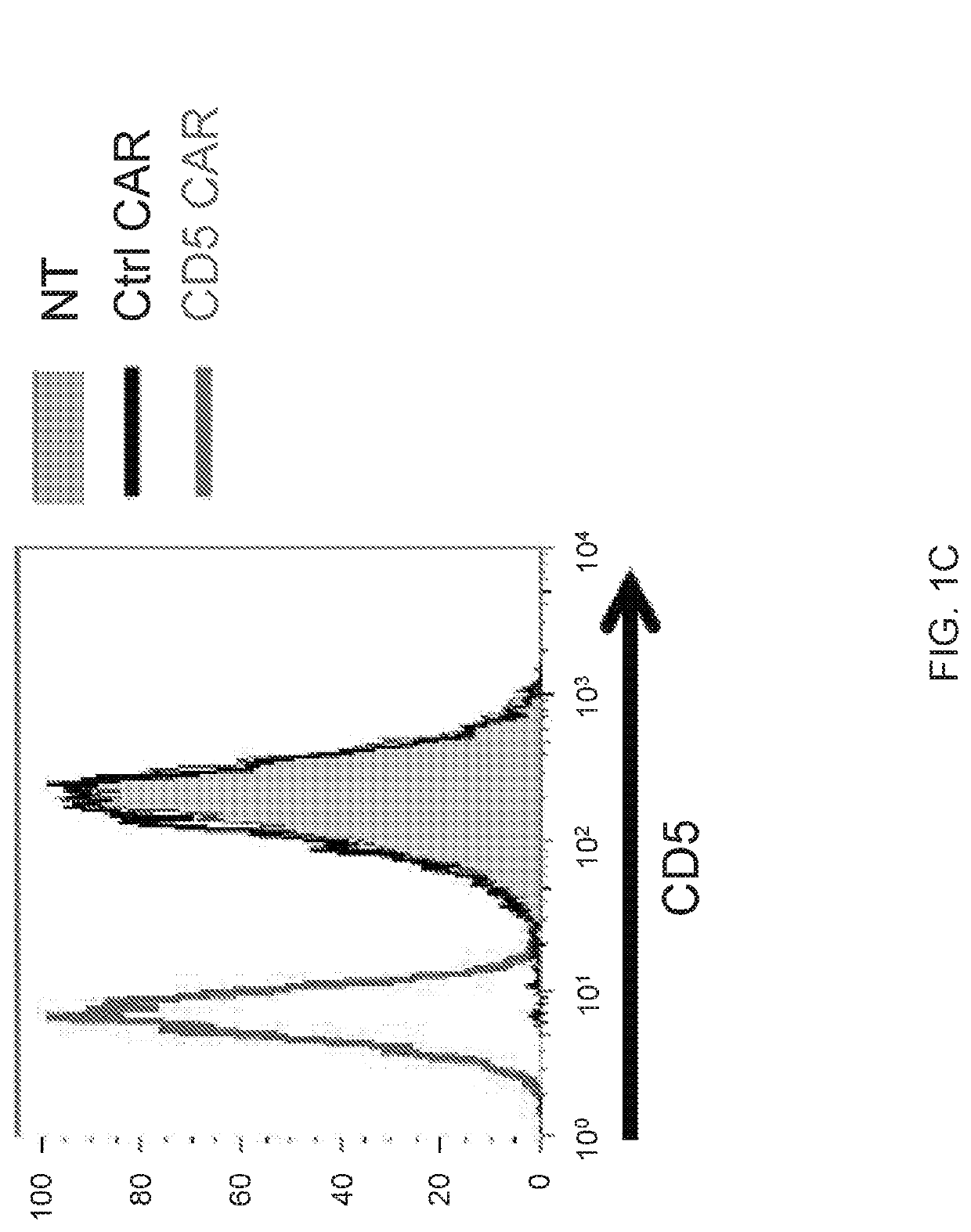
ActiveUS10786549B2Increased persistenceImprove responsePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD5Antigen receptors
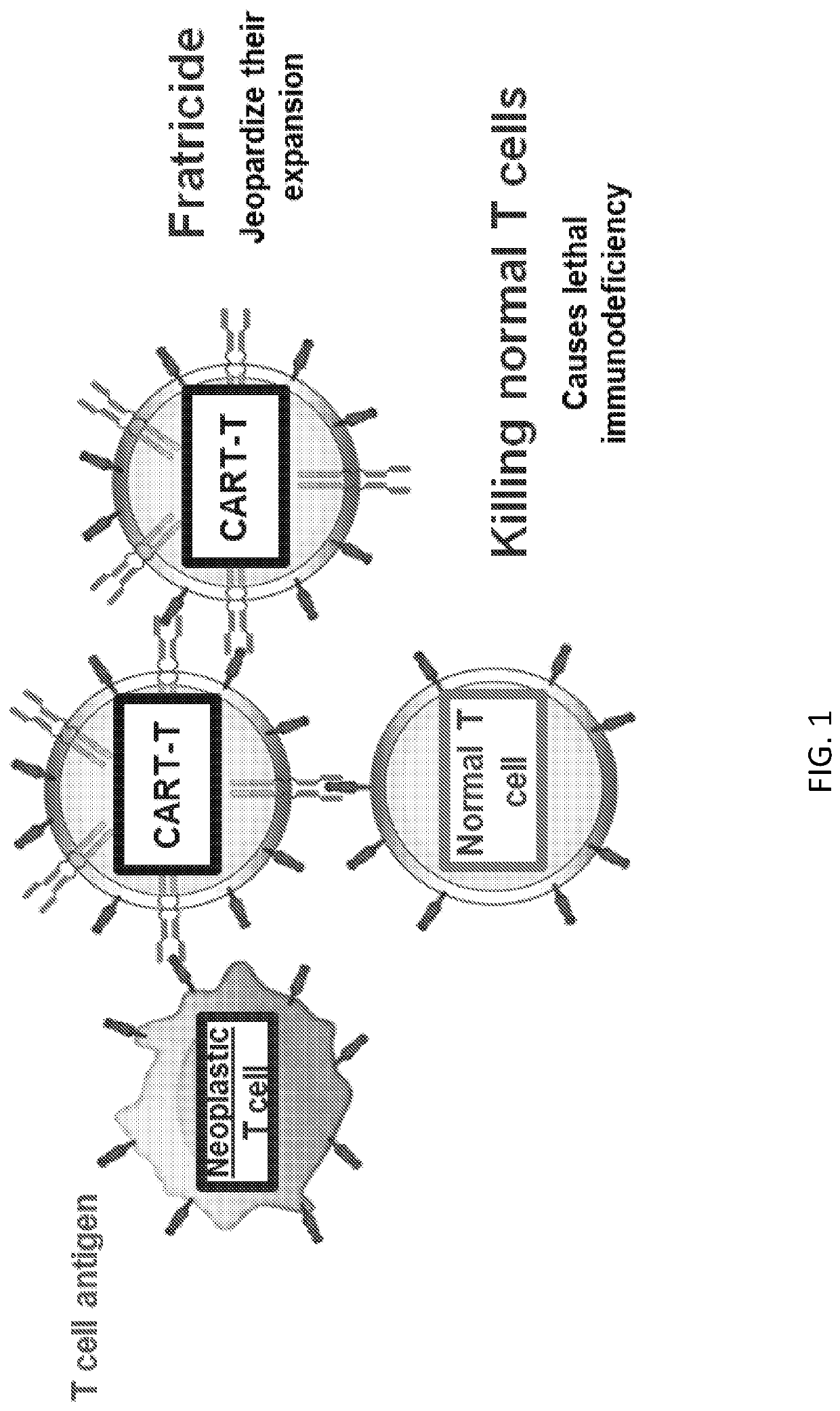
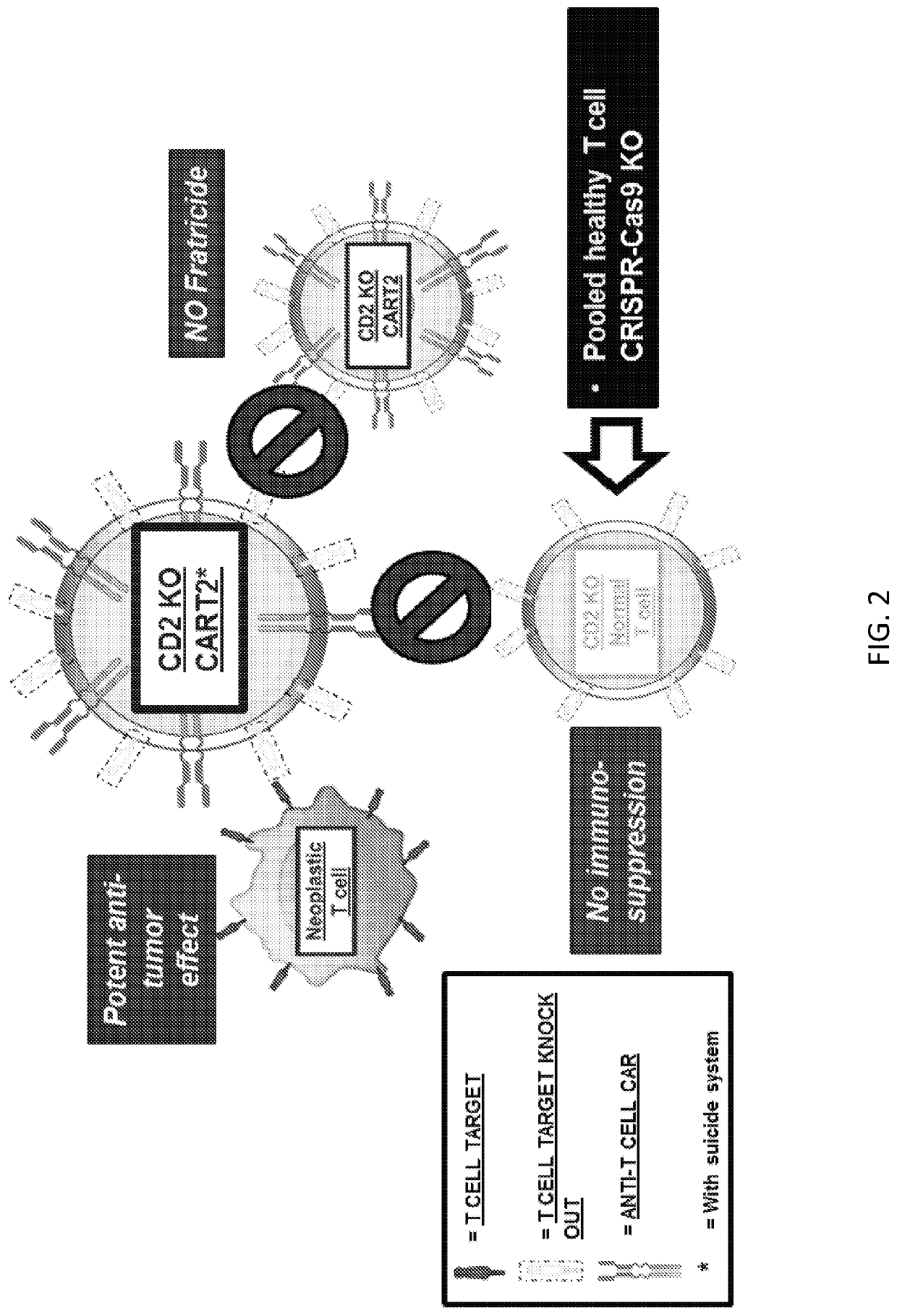
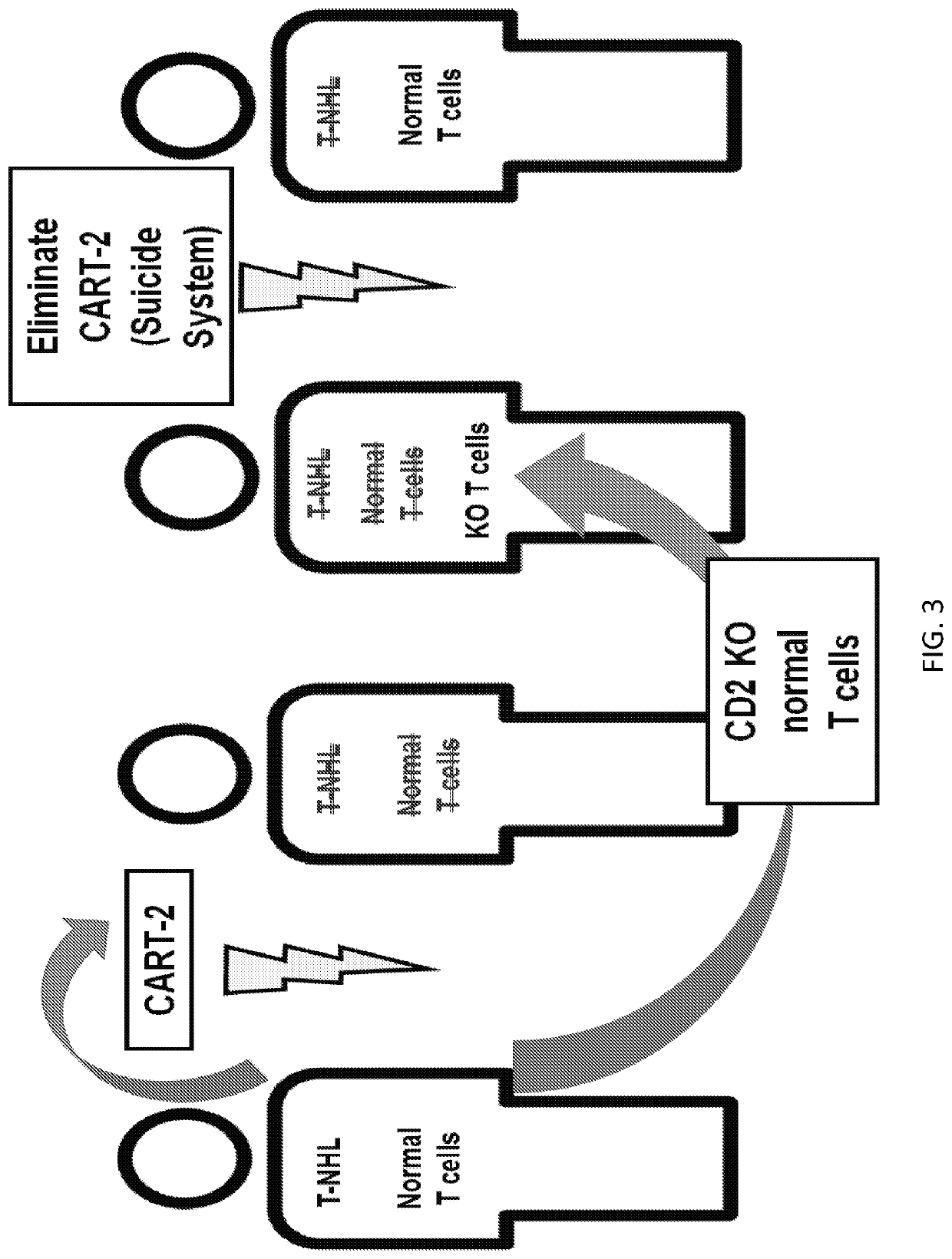
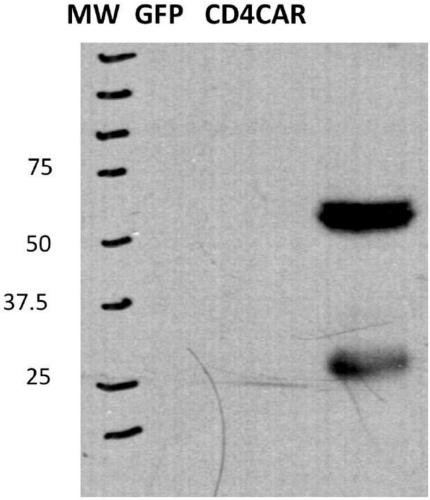
Embodiments of the disclosure include methods and compositions related to immunotherapy that targets CD5. In particular embodiments, immune cells engineered to comprise a chimeric antigen receptor (CAR) that targets CD5 are contemplated, and uses thereof. In particular embodiments, the immune cells expressing the CAR do not commit fratricide to any great extent against T cells that express CD5 and which are endogenous to an individual receiving the immune cells.
Owner:BAYLOR COLLEGE OF MEDICINE
Soluble protein CD5 or CD6 for the treatment of cancer or tumor or for use as an adjuvant
InactiveUS9445582B2Organic active ingredientsCell receptors/surface-antigens/surface-determinantsDiseaseAdjuvant
The present invention discloses soluble isoforms of CD5 and CD6, which are scavenger-like lymphocyte receptors, for use in the prophylaxis or therapy of disorders or in therapeutic interventions, which would benefit from an exacerbated immune response to either endogenous or exogenous antigens, resulting from a decrease in lymphocyte subpopulations with regulatory functions and / or increase in lymphocyte subpopulations with effector functions. Special disorders are cell growth disorders, and chronic infections of bacterial, viral, fungal or parasitic origin. The invention also provides animal models for the study and the prophylaxis / treatment of autoimmune diseases, cancer, and chronic infections.
Owner:FUNDACIO CLINIC PER A LA RECERCA BIOMEDICA +2
Human T lymphoblastic leukemia/lymphoma cell strain and application thereof
ActiveCN112877290AIn vitro shape stabilityIn line with clinical tumor biological characteristicsMicrobiological testing/measurementDrug screeningT-lymphoblastic lymphoma/leukemiaCD5
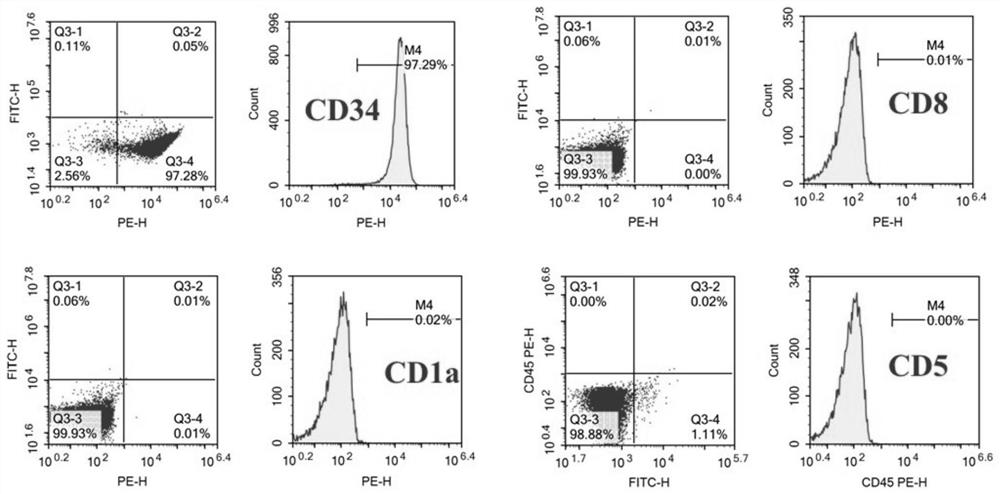
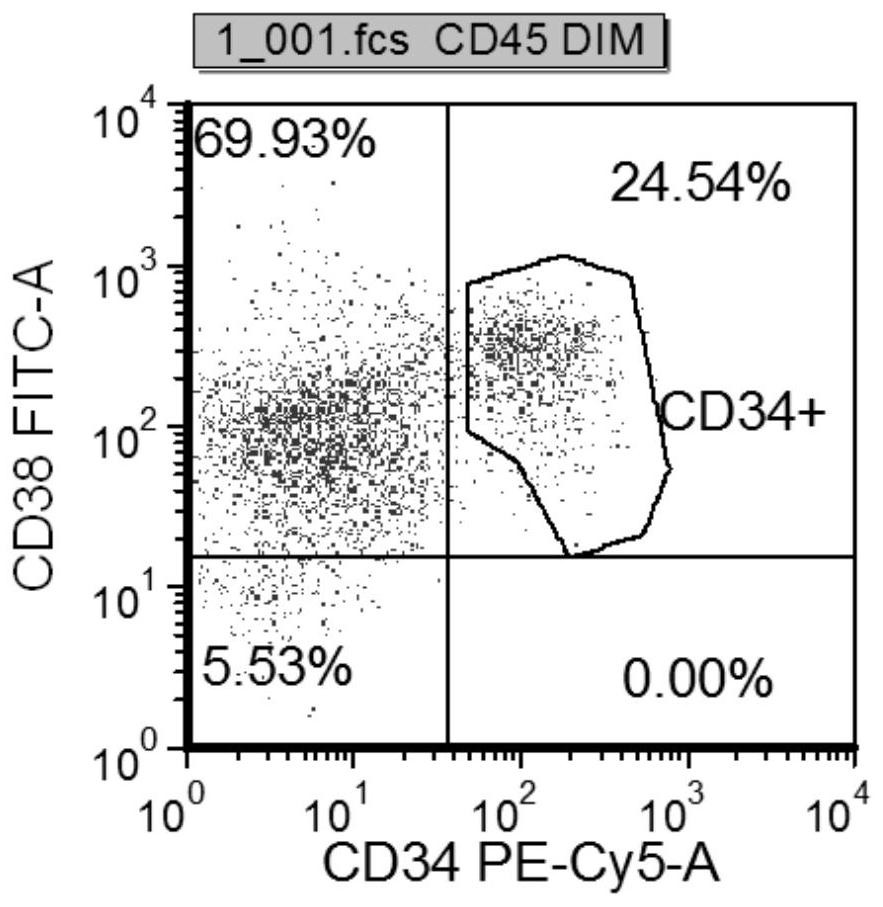
The invention discloses a human T lymphoblastic leukemia / lymphoma cell strain as well as a construction method and an application thereof. The human T lymphoblastic leukemia / lymphoma cell strain is named as human T lymphoblastic leukemia / lymphoma ZYXY-T1, and is preserved in the China Center for Type Culture Collection (Wuhan University, Wuhan, China) on January 20, 2021, and the preservation number is CCTCC NO: C202143. According to the invention, mononuclear cells are extracted and separated from peripheral blood of a clinical human T lymphoblastic leukemia / lymphoma patient, and are subjected to in-vitro culture and continuous natural passage to obtain the T lymphoblastic leukemia / lymphoma protein. The leukemia cell strain has the typical surface antigen expression characteristic of ETP-ALL, namely, the leukemia cell strain does not express CD1alpha, CD5 and CD8, highly expresses a dry line marker CD34, has good in-vitro proliferation capacity and in-vivo tumorigenesis capacity, can be used as a cell material for researching the occurrence and development mechanism of ETP-ALL and individualized treatment in-vitro research, and can be used for in-vivo and in-vitro research of ETP-ALL drug screening, evaluation, and clinical medication.
Owner:ZHEJIANG UNIV
Spleen regulating type B lymphocyte as well as preparation method and application thereof
ActiveCN114686427AGood adoptive transferReduce fibrosisCell dissociation methodsMammal material medical ingredientsSpleen cellCD1D
The invention provides a spleen regulating type B lymphocyte as well as a preparation method and application thereof. On one hand, the invention provides a spleen regulating type B lymphocyte, wherein the proportion of CD1d, CD5 and CD21 / 35 antibody positive regulating type B lymphocytes in the cell is more than 94.6%. The invention further provides a method for preparing the spleen regulating type B lymphocyte. The method for preparing the spleen regulating type B lymphocyte comprises the following steps: taking a spleen cell suspension, and marking with surface antibodies CD1d, CD5 and CD21 / 35; the labeled cells are sorted by fluorescence activated cell sorting. The third aspect of the invention provides application of the spleen regulating type B lymphocyte in preparation of a preparation for targeted colonization in bilateral kidneys. The fourth aspect of the invention provides application of the spleen regulating type B lymphocyte in preparation of a product for kidney protection.
Owner:THE FIRST MEDICAL CENT CHINESE PLA GENERAL HOSPITAL
Compositions and methods for the depletion of cd5+ cells
InactiveUS20200276326A1Reduce and prevent likelihoodReduce the possibilityOrganic active ingredientsDipeptide ingredientsAutoimmune conditionCD5
The invention provides anti-CD5 antibodies, antigen-binding fragments thereof, and antibody drug conjugates thereof, for use in treating, for example, a stem cell disorder, cancer, or autoimmune disease, among other hematological and proliferative diseases. Compositions and methods for depleting populations of CD5+ cells, such as CD5+ cancer cells and CD5+ immune cells are described, and can be used to treat cancers and autoimmune diseases directly as stand-alone therapies by eradicating cancerous cells and autoreactive immune cells that express CD5 and / or to prepare a patient for hematopoietic stem cell transplantation, for instance, by depleting populations of CD5+ immune cells that cross-react with, and mount an immune response against, non-self hematopoietic stem cells.
Owner:MAGENTA THERAPEUTICS INC
A kind of human t-lymphoblastic leukemia/lymphoma cell line and its application
ActiveCN112877290BIn vitro shape stabilityIn line with clinical tumor biological characteristicsMicrobiological testing/measurementDrug screeningCD5CD8
The invention discloses a human T-lymphoblastic leukemia / lymphoma cell line and its construction method and application. The human T-lymphoblastic leukemia / lymphoma cell line is named as human T-lymphoblastic leukemia / lymphoma ZYXY-T1, and was deposited in the China Center for Type Culture Collection (Wuhan University, Wuhan, China) on January 20, 2021, with a deposit number It is CCTCC NO:C202143. The present invention is obtained by extracting and separating mononuclear cells from the peripheral blood of clinical human T-lymphoblastic leukemia / lymphoma patients, and culturing them in vitro for continuous natural passage. This leukemia cell line has the typical surface antigen expression characteristics of ETP‑ALL, that is, it does not express CD1α, CD5, CD8, highly expresses the stemness marker CD34, has good in vitro proliferation ability and in vivo tumorigenesis ability, and can be used as a study on the development mechanism of ETP‑ALL Research and individualized treatment In vitro research cell materials can also be used for in vivo and in vitro research ETP‑ALL drug screening, evaluation, and guidance for clinical medication.
Owner:ZHEJIANG UNIV
Method of predicting response of a human subject suffering from multiple sclerosis to interferon beta, (ifn-ß)
InactiveUS20170248617A1Easy and reliable in vitroPeptide/protein ingredientsDisease diagnosisTotal countLymphocyte
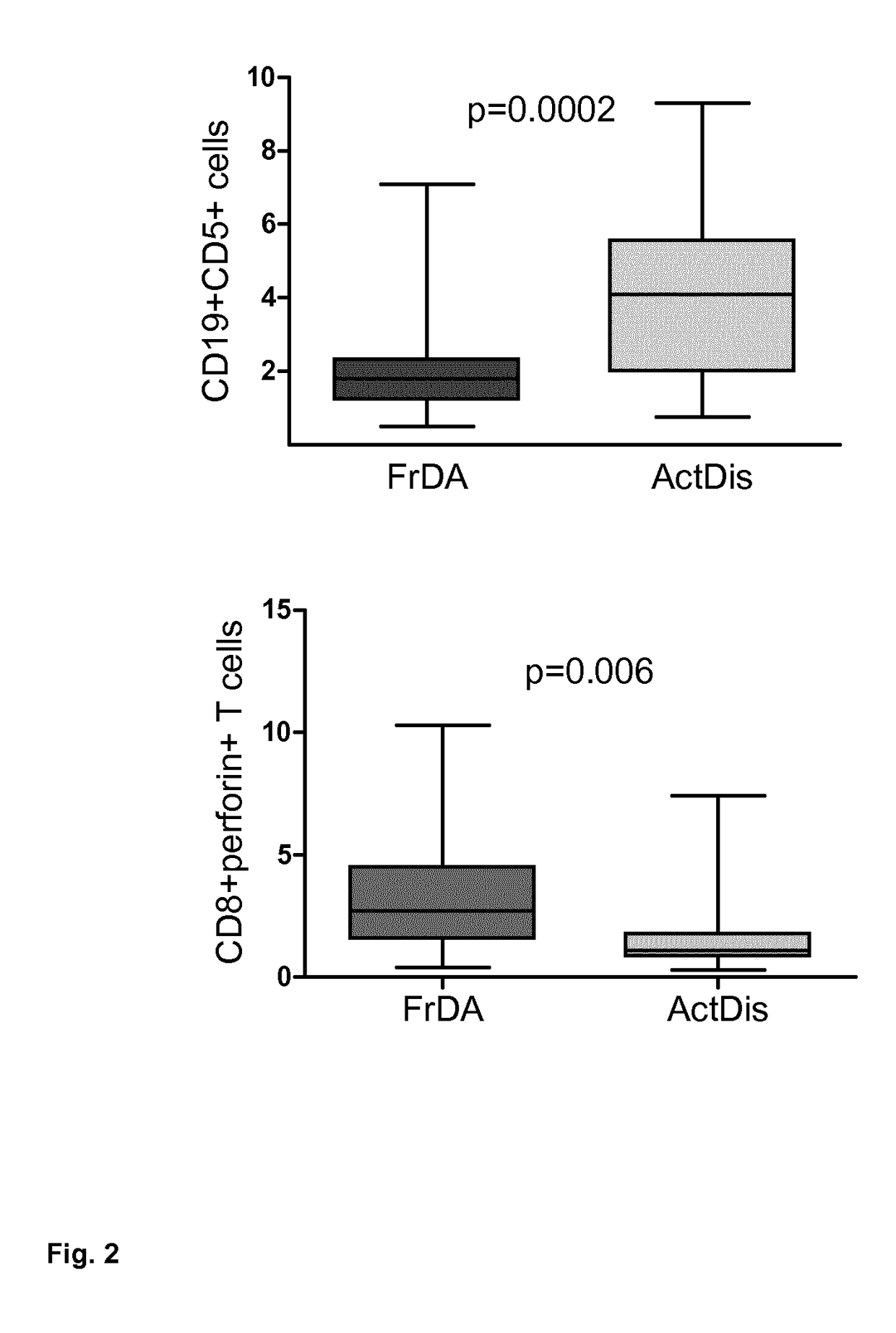
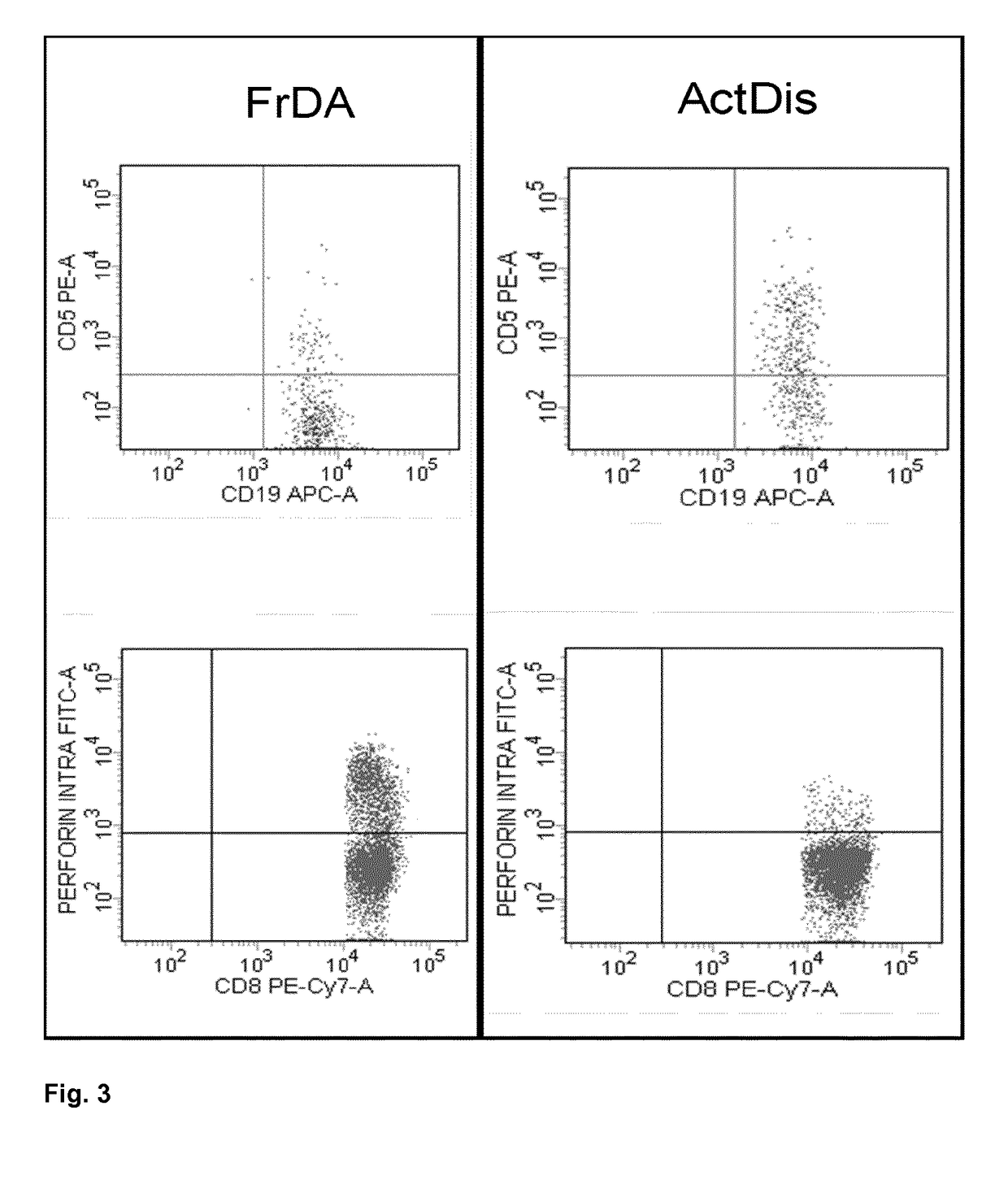
The present invention refers to a method of predicting response of a human subject to Interferon beta, (IFN-β), wherein the subject is suffering from Multiple Sclerosis (MS), and wherein the method comprises using, as an indicator, the percentage of CD5+ CD19+ CD45+ B cells over the total count of lymphocytes (CD45+ cells) in a biological sample originating from the human subject and the percentage of CD8+ CD45+ perforin+ T cells over the total count of lymphocytes (CD45+ cells) in a biological sample originating from the human subject, wherein if the percentage of CD5+ CD19+ CD45+ B cells over the total count of lymphocytes (CD45+ cells) is lower than or equal to 3% and / orthe percentage of CD8+ CD45+ perforin+ T cells over the total count of lymphocytes (CD45+ cells) is greater than or equal to 1%, is indicative of response.
Owner:FUNDACION PARA LA INVESTIGACION BIOMEDICA DEL HOSPITAL UNIVRIO RAMON Y CAJAL
Combination reagent and system for detecting acute myeloid leukemia cells
ActiveCN109655616BWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD5Antibody expression
The invention relates to a combined reagent and system for detecting acute myeloid leukemia cells, belonging to the technical field of medicine. The combination reagent includes at least one of the following antibody combinations: antibody combination 1, including CD38, CD13, CD34, CD117, CD33, CD19, HLA‑DR and CD45 antibodies; antibody combination 2, including CD38, CD64, CD34, CD123 , CD56, CD14, HLA‑DR, and CD45 antibodies; antibody panel 3: includes CD38, CD7, CD34, CD5, CD11b, CD15, and CD45 antibodies. The antibody combination of the present invention covers the expression markers of three lineages of granulocystosis, monocystosis and lymphoma, and with the establishment of a normal antibody expression pattern, it can identify tumor cells to the greatest extent. Moreover, a large amount of experimental data shows that there is no problem of mutual inhibition of expression among the antibodies in each combination of the present invention. AML‑MRD can be detected comprehensively, quickly and with high sensitivity through multiparameter flow cytometry analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
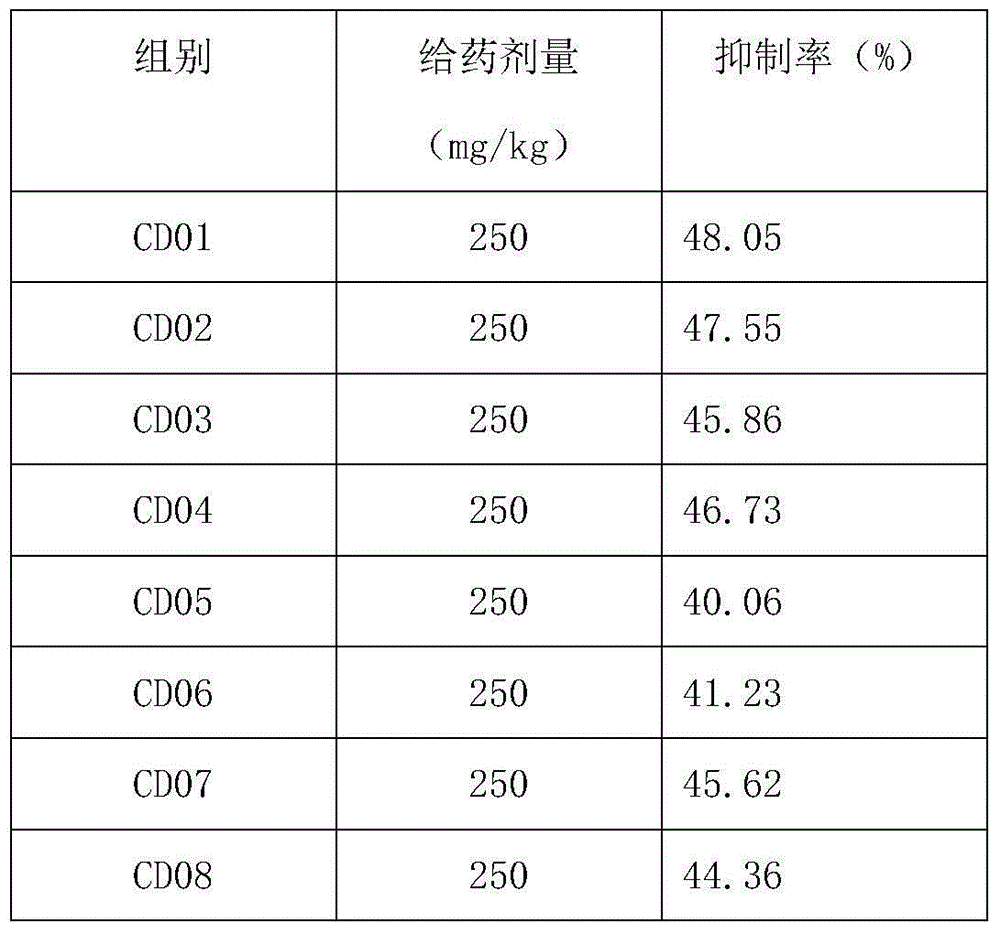
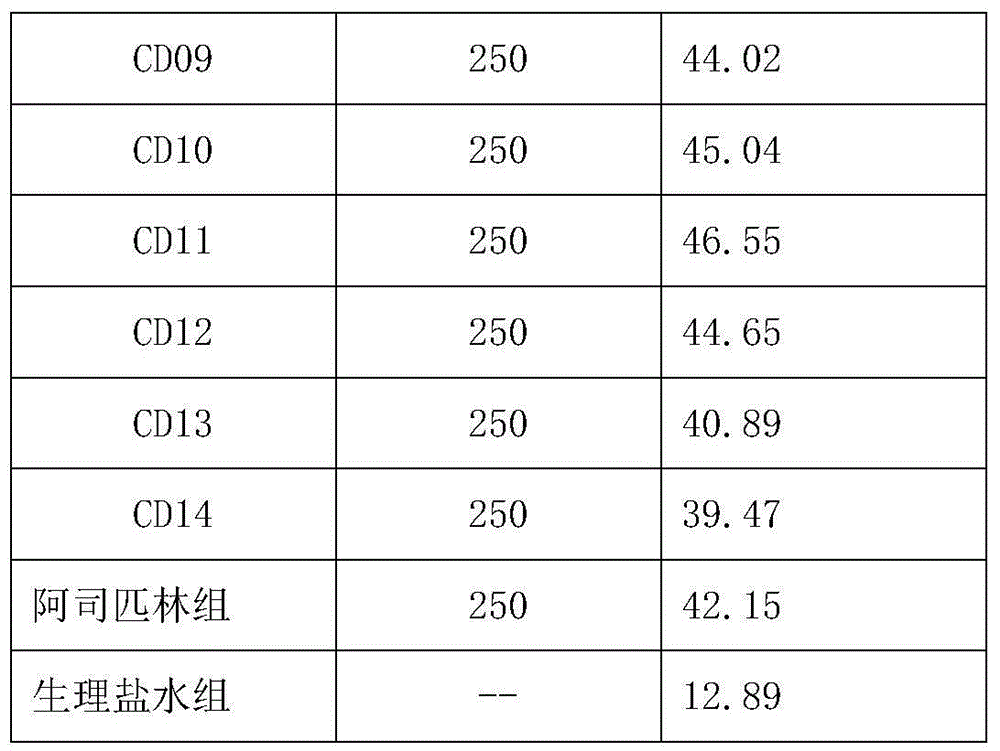
Use of oviductus ranae in preparing medicament for curing and preventing diseases caused by immunologic dysfunction
InactiveCN101637478AImmunosuppressionInhibit inflammationNervous disorderAntipyreticDiseaseAutoimmune responses
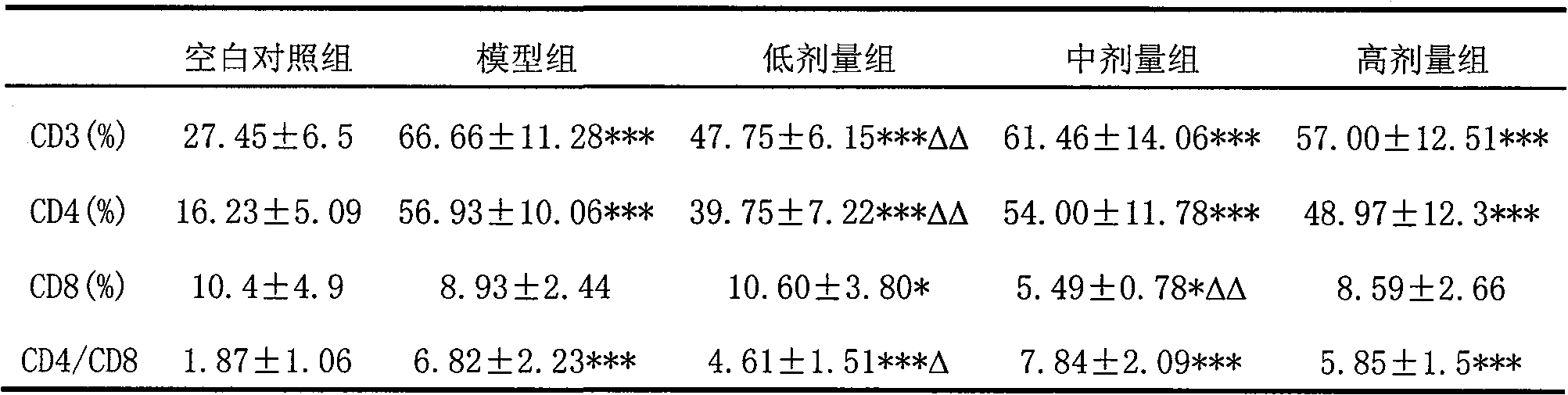
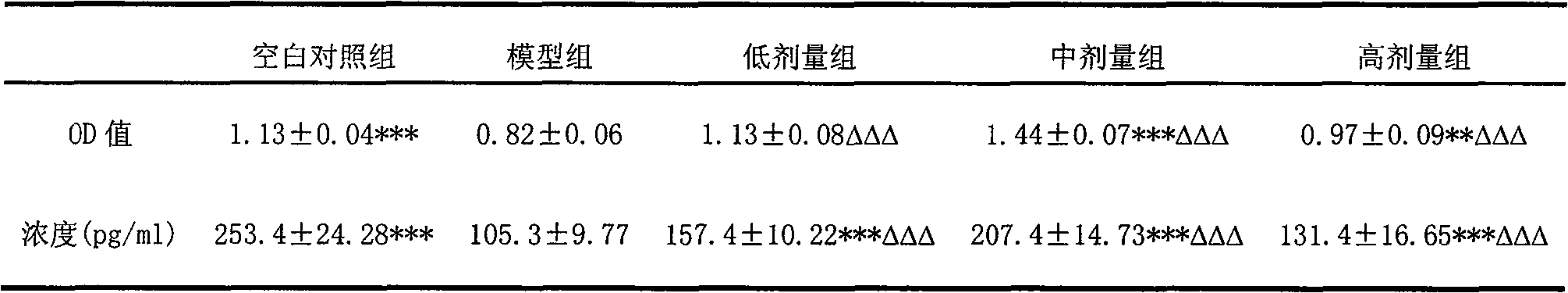
The invention relates to use of oviductus ranae in preparing medicament for curing and preventing diseases caused by immunologic dysfunction. The flow cytometry and the ELISA method proves that the oviductus ranae has a function for immunity adjustment by the results of mouse spleen T-lymphocyte subsets CD3, CD4, CD5, CD8 and the ratio of CD4 / CD8, namely, when the immunity is high, the oviductus ranae has a function for weakening the immunity, and when the immunity is low, the oviductus ranae has a function for enhancing the immunity. The new use of the oviductus ranae proves that the oviductus ranae has a therapeutical effect for the diseases caused by the immunologic dysfunction such as asthma, autoimmune disease, hepatitis and tumor, thereby developing the use of the oviductus ranae inpreparing medicament for curing and preventing the diseases caused by the immunologic dysfunction.
Owner:CHANGCHUN UNIV OF CHINESE MEDICINE
Compositions and methods for the depletion of cd5+ cells
PendingUS20210369854A1Reduce and prevent likelihoodReduce the possibilityOrganic active ingredientsDipeptide ingredientsAutoimmune conditionAntiendomysial antibodies
The invention provides anti-CD5 antibodies, antigen-binding fragments thereof, and antibody drug conjugates thereof, for use in treating, for example, a stem cell disorder, cancer, or autoimmune disease, among other hematological and proliferative diseases. Compositions and methods for depleting populations of CD5+ cells, such as CD5+ cancer cells and CD5+ immune cells are described, and can be used to treat cancers and autoimmune diseases directly as stand-alone therapies by eradicating cancerous cells and autoreactive immune cells that express CD5 and / or to prepare a patient for hematopoietic stem cell transplantation, for instance, by depleting populations of CD5+ immune cells that cross-react with, and mount an immune response against, non-self hematopoietic stem cells.
Owner:HEIDELBERG PHARMA RES GMBH
Detection of CD5 and methods and compositions for modulating CD5
ActiveUS11369663B2Peptide/protein ingredientsMammal material medical ingredientsAutoimmune conditionCD5
Owner:WASHINGTON UNIV IN SAINT LOUIS
Anti-cd5 protein monoclonal antibody and its cell line, preparation method and application
ActiveCN113105547BStrong specificityIncreased sensitivityBiological material analysisMicroorganism based processesImmunological testingCD5
The invention relates to a monoclonal antibody capable of recognizing human CD5 antigen, a secreting cell line, its preparation method and its application in immunoassay. The above technical scheme selects the 398th to 495th amino acid at the C-terminus of the CD5 protein as the antigenic peptide, and performs codon optimization to become a gene fragment suitable for expression in Escherichia coli BL21. The resulting recombinant protein contains the CD5 protein fragment and histidine protein tag. The recombinant protein is used to immunize mice, and through cell fusion, screening and subcloning, the mouse hybridoma cell line 11H3 that efficiently secretes anti-CD5 protein monoclonal antibody, and the anti-CD5 protein monoclonal antibody secreted by the cell line are obtained Antibody. The antibody obtained by this protocol has high specificity and sensitivity, can specifically recognize cells expressing CD5 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Use of Anti-cd5 antibody drug conjugate (ADC) in allogeneic cell therapy
PendingUS20210355230A1Easy to acceptPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAutoimmune conditionCD5
The invention provides methods of depleting CD5+ cells in human patients undergoing chimeric antigen receptor (CAR) immunotherapy in order to promote acceptance of CAR expressing immune cells. Anti-CD5 antibody drug conjugates (ADCs) are administered as a conditioning regimen to a human patient receiving autologous or allogeneic CAR expressing immune cells such that the CAR expressing immune cells are accepted by the human patient. Compositions and methods of the invention can be used in combination with CAR therapy to treat a variety of pathologies, including autoimmune diseases and cancer.
Owner:HEIDELBERG PHARMA RES GMBH
Mouse lymphoma cell line and animal model of human high grade b-cell lymphoma
The invention relates to a new, spontaneous mouse lymphoma cell line displaying CD19, B220, MHC II, surface IgG2a / kappa chain and MAC-1, which is negative for CD5, animal models of B-cell lymphoma based on said cell line and methods for assessing lymphoma propagation and lymphoma expansion based on said cell line.
Owner:PECSI TUDOMANYEGYETEM
Chimeric antigen receptor targeting CD5 and its application
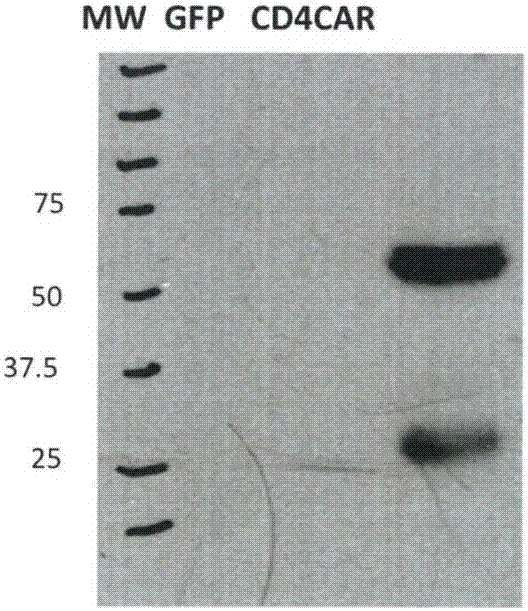
ActiveCN109266667BGood killing effectNo lethal effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD5Natural Killer Cell Inhibitory Receptors
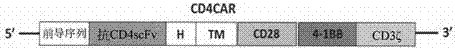
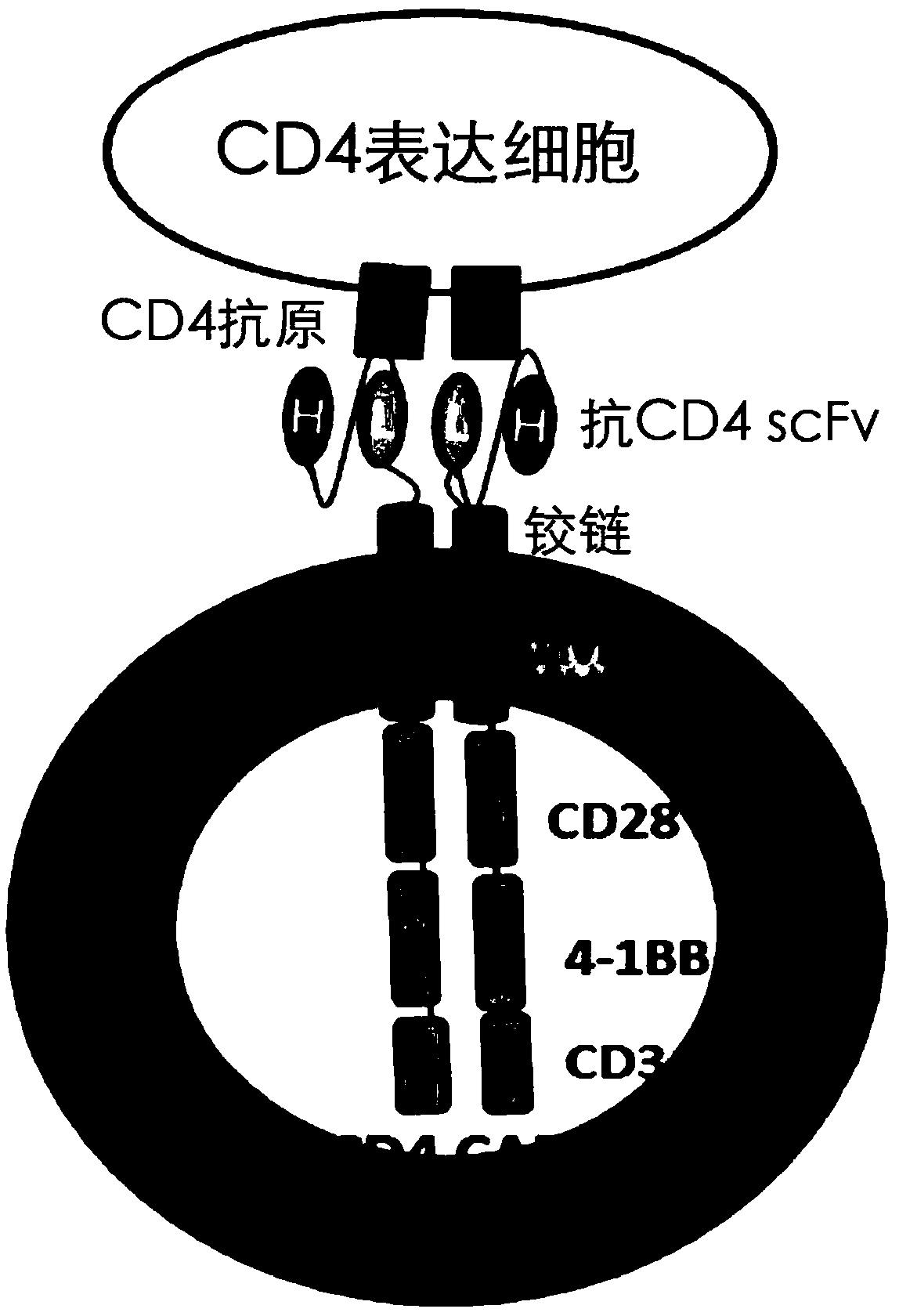
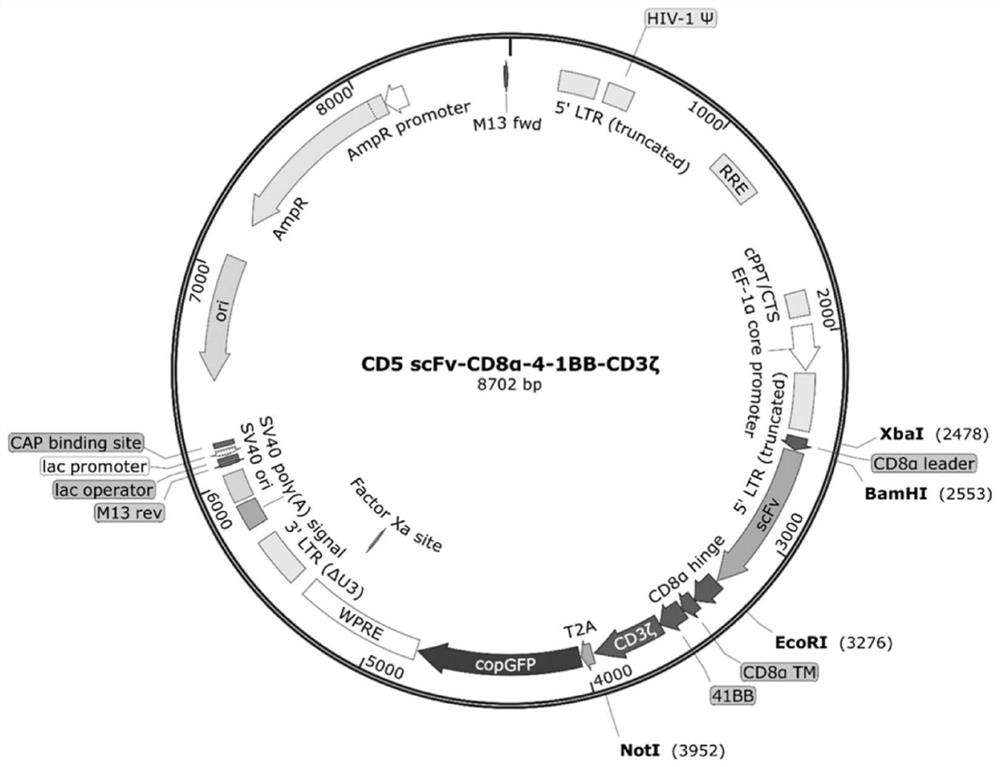
The present invention provides a nucleic acid molecule encoding a chimeric antigen receptor targeting CD5, the chimeric antigen receptor comprises an extracellular region, a transmembrane region and an intracellular signal transduction region, the extracellular region encoded by it The region comprises a CD5 binding domain, and the CD5 binding domain is the amino acid sequence shown in SEQ ID NO.3. The cytokines secreted by NK cells were detected by flow cytometry, degranulation analysis experiments, and ELISA, which proved that the CD5 CAR NK cells had a strong killing effect on blood tumor cells expressing CD5, but had a strong killing effect on cells that did not express CD5. Weak, effectively preventing off-target effects. The chimeric antigen receptor CD5 scFv-CD8α-4-1BB-CD3ζ of the present invention can be used for the treatment of CD5-positive lymphocytic blood tumors.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Combined reagent and system for evaluating the prognosis of chronic lymphocytic leukemia
The invention relates to a combined reagent and system for evaluating the prognosis of chronic lymphocytic leukemia, belonging to the field of medical technology detection. The combination reagent includes at least one of the following antibody combinations: antibody combination 1, including CD5, IgG1, CD3, CD19 and CD45 antibodies; antibody combination 2, including CD5, ZAP70, CD3, CD19 and CD45 antibodies; antibody combination 3: Includes CD38, CD49d, CD19, CD5, and CD45 antibodies. Among the above-mentioned antibody combinations against CLL of the present invention, the expressions of ZAP70, CD38, and CD49d are of great value in evaluating prognosis, and ZAP70 is one of the more accurate ones, but its clinical application is limited due to intracellular staining and gating techniques, and this The invention solves this problem to the greatest extent. In addition, two indicators are added. Combining these markers can provide a more comprehensive assessment of the prognosis of chronic lymphocytic leukemia.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
T cell depletion therapy
Provided herein are methods of depleting T cells for therapeutic use comprising administering an anti-CD2 or anti-CD5 antibody drug conjugate (ADC) for therapy. Anti-CD2 ADCs or anti-CD5 ADCs are provided for use as agents for the treatment of stem cell disorders, cancer or autoimmune diseases, as well as other hematological and proliferative diseases. The described compositions and methods can be used to deplete a population of CD2 + or CD5 + cells, such as CD2 + or CD5 + cancer cells or CD2 + or CD5 + immune cells, and can also be used to prepare for hematopoietic stem cell transplantation or solid organ transplantation for a patient.
Owner:美真达治疗公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com