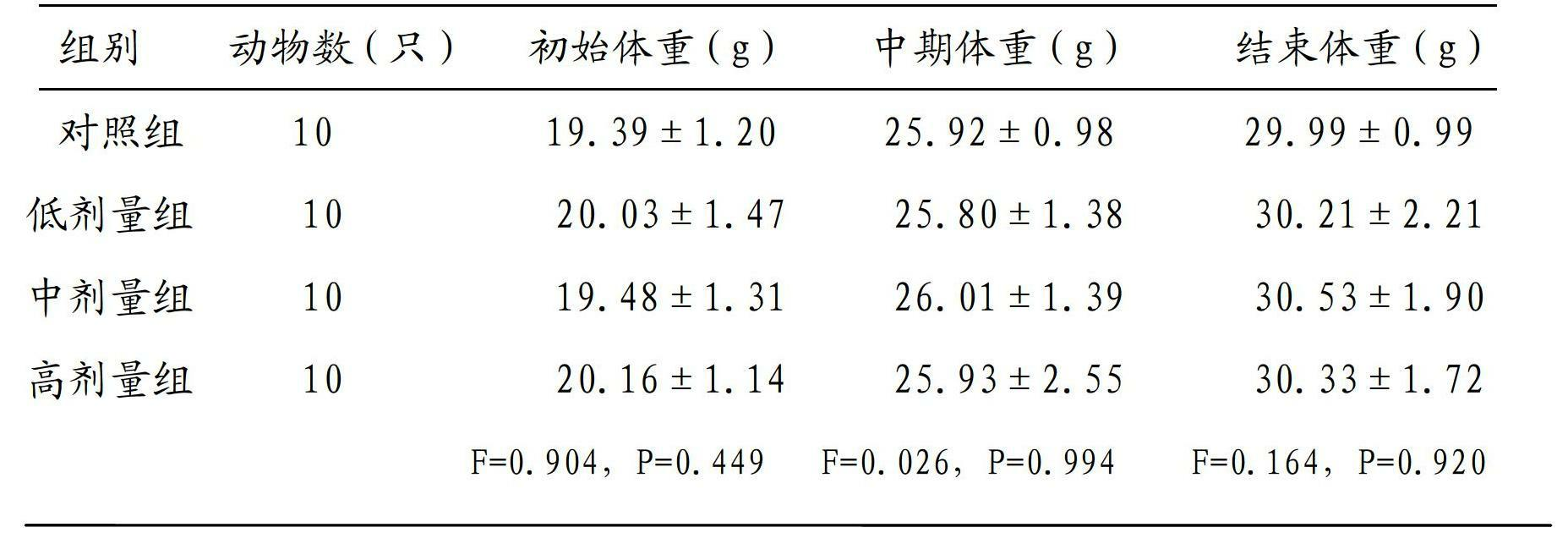
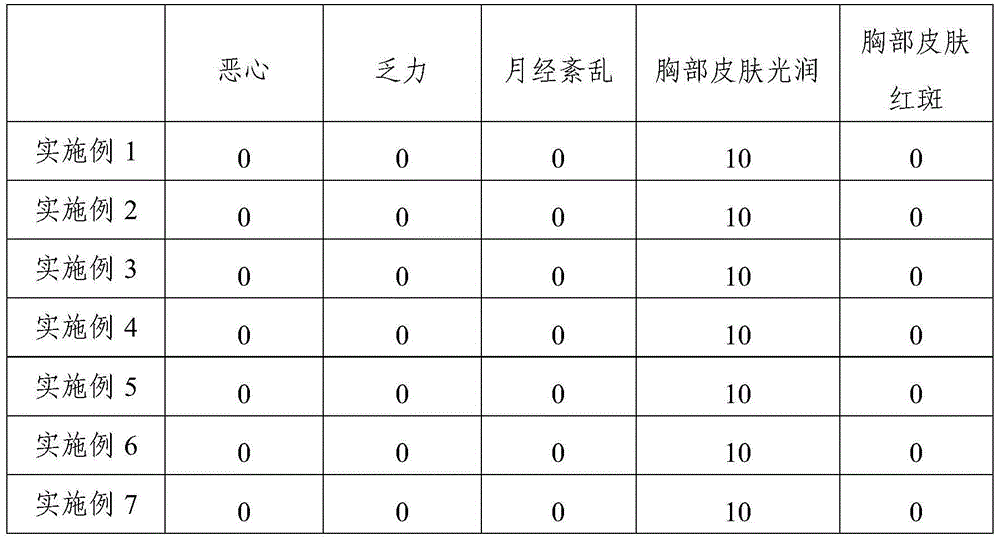
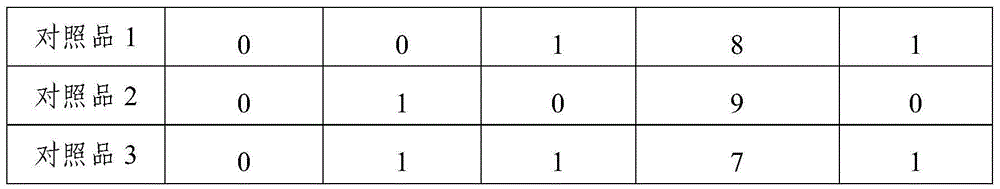
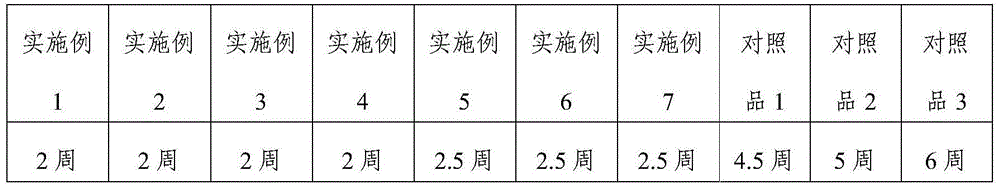
Patents
Literature
84 results about "Oviductus Ranae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hasma (Harsmar, Hashima) is a Chinese and widely Central Asian dessert ingredient made from the dried fatty tissue found near the fallopian tubes of true frogs, typically the Asiatic Grass Frog (Rana chensinensis).Because of its whitish appearance, Hasma is often mistakenly described as "snow frog fat". The Western pharmaceutical term is Oviductus Ranae
Micrometer sterilized Oviductus Ranae powder, preparation method and application thereof
InactiveCN102697813ASmall particlesAddresses Susceptibility to Microbial ContaminationAmphibian material medical ingredientsPowder deliveryPreservativePharmaceutical medicine
The invention discloses a micrometer sterilized Oviductus Ranae powder with particle diameter of 0.1-500 micrometer. The Oviductus Ranae powde is prepared from low-temperature sterilized Oviductus Ranae by ultra-micronizing. The invention also discloses preparation method and application of the Oviductus Ranae powder. The invention combines low-temperature sterilizing and ultra-micronizing to produce pure, preservative-free and easily-absorbable fine micrometer Oviductus Ranae powder in large scale, and the preparation method facilitates mass production of high-quality pharmaceutical, food or health food preparation.
Owner:INFINITUS (CHINA) CO LTD
Oviductus ranae and processing method thereof as well as oviductus ranae beverage
The invention relates to the field of food industry and discloses oviductus ranae and a processing method thereof as well as an oviductus ranae beverage. The processing method provided by the invention comprises the following steps of: preprocessing dry oviductus ranae, and then soaking in boiled water till temperature is less than or equal to 70 DEG C; adding alcohol and ginger, both of which account for 4-6% of the dry oviductus ranae by weight, and stirring for 4-5hours; keeping the temperature at 50-70 DEG C while stirring; after stirring, removing the ginger and impurities; and crushing and sterilizing, thereby obtaining suspension, namely, oviductus ranae. According to the processing method provided by the invention, the traditional high-temperature stewing processing method is changed, and the boiled water is utilized to soak and the alcohol and ginger are added at a suitable temperature, so that the loss of active matters of the oviductus ranae is reduced, the taste is improved, the fishy smell is removed and the effect of the oviductus ranae is increased.
Owner:BAISHAN LINYUANCHUN ECOLOGY TECH
Protein nutritive jellies prepared from oviductus ranae enzymatic hydrolysate and byproduct
The invention belongs to the technical field of health-care food, and particularly relates to an oviductus ranae functional peptide nutritive jelly and an oviductus ranae enzymolysis byproduct protein nutritive jelly. The jellies adopt oviductus ranae functional peptide and unhydrolyzed oviductus ranae as protein enhancers and the mixture of carrageenan, gelatin and xanthan as gelatinizer, white granulated sugar, citric acid, potassium sorbate and essence are applied to regulate the flavor, the products are clear in color and transparent, the taste is delicate, the elasticity is moderate, and the jellies are chewy; the hardness of the textures is moderate, and air bubbles do not exist; the taste and the smell are moderately sour and sweet, and the jellies do not have the fishy smell of oviductus ranae. The preparation process for the jellies mainly includes two parts, i.e. the preparation of the oviductus ranae functional peptide and the preparation of the protein nutritive jellies. The inspected quality indexes of the protein nutritive jellies disclosed by the invention fully meet product standards, and since the protein nutritive jellies respectively contain the oviductus ranae functional peptide and the collagen, the protein nutritive jellies have the effects of enhancing the immunity of the human body, reducing blood fat and blood sugar, maintaining beauty and the like, and is significant in guaranteeing the physical health of people.
Owner:JILIN UNIV
Medicine composition for relieving eyestrain
InactiveCN103417733AImprovement of eye problemsImprove systemic symptomsAmphibian material medical ingredientsSenses disorderLuteinVitamin A Alcohol
The invention relates to a medical preparation, in particular to a medicine composition for relieving eyestrain. The effective constituents of the composition are composed of, by weight, 0.01 part to 0.3 part of oviductus ranae, 0.01 part to 0.03 part of xanthin, 0.01 part to 0.03 part of vitamin A, and 1 part to 5 parts of water extract containing fructus lycii, folium mori, semen cassiae, blueberries and Cassia tora, wherein the weight of fructus lycii, the weight of folium mori, the weight of semen cassiae, the weight of the blueberries and the weight of Cassia tora are the same. The composition can resist eyestrain and is suitable for people with computer eyestrain syndromes, VDT eyestrain, computer vision syndromes, computer terminal syndromes, eyestrain and the like.
Owner:广东南方国晟生命科技有限公司
Instant oviductus ranae and preparation method thereof
The invention provides instant oviductus ranae, which takes oviductus ranae as raw materials, and is prepared through a method as follows: purifying and deodorizing the oviductus ranae, curing at high temperature, sterilizing, freezing, vacuum drying, and preparing to be instant oviductus ranae food. The instant oviductus ranae is convenient to eat, is easy to store, is convenient to carry, has no additive, and is safe to eat. The invention also provides a preparation method of the instant oviductus ranae. The method is simple in process without using food additives.
Owner:桦甸市吉元土产有限公司
Panax quinguefolium snow-frong thick soup and its preparation method
The invention relates to a thick soup containing Panax guingnefolium and Oviductus Ranae, and the preparation method. The invention belongs to the nutritive heathy product field. The thick soup is characterized in that the thick soup is porridge, which mainly comprises Panax guingnefolium, forest frog oil, Lycium chinense Miller, fructus jujubae, etc. as the raw materials. The preparation process comprises following steps: cleaning dry or fresh forest frog oil, removing impurities, soaking with water 7 to 40 times of the weight of the forest frog oil for 0.6 to 12 h, and steaming for 10 to 15 min; slicing Panax guingnefolium, mixing with Lycium chinense Miller and fructus jujubae, and steaming in a container; and adding water into the product, mixing uniformly, boiling, and canning to obtain the final product. The invention aims to solve the problems of difficult storage of forest frog oil, fishy smell of porridge, unreasonable nutrition arrangement, etc.
Owner:于晶
Preparation method for instant oviductus ranae particle
The invention discloses a preparation method for an instant oviductus ranae particle. The preparation method is characterized by comprising the following steps of: A, preparing ginseng extract fine powder: performing reflux extraction on ginseng by using ethanol for three times, combining filtrates, recovering ethanol, concentrating into thick paste, adding water and alcohol for depositing, concentrating a supernatant into thick paste, and drying to obtain ginseng extract fine powder; B, preparing oviductus ranae submicron powder: removing tendons and membranes from oviductus ranae, drying, and smashing into a nanometer grade particle to obtain the oviductus ranae submicron powder; and C, forming particle: uniformly mixing the ginseng extract fine powder, the oviductus ranae submicron powder and other pharmaceutically-acceptable carriers, adding ethanol for pelletizing, drying, and pelletizing to obtain a finished product. In the method, Jilin ginseng is preferable. Due to the adoption of the preparation method, the product has the advantages of remarkable increase in the dissolution rate, increase in nutrient absorbing rate of a human body, quick response, high bioavailability, easiness for operating a process, no need of special equipment or chemical reagent, safe production, basic and stable process conditions, solving of 'three wastes' treatment problem, and suitability for industrial mass production.
Owner:JILIN HUAKANG SHIYUAN BIOTECH
Composite glycopeptide breast beauty cream and preparation method thereof
InactiveCN104398399APromote transdermal absorptionQuick Breast EnhancementCosmetic preparationsToilet preparationsGlycopeptideOviductus Ranae
The invention relates to a composite glycopeptide breast beauty cream, which is prepared from the following raw materials by weight: 0.1-0.5 part of sheep placenta protein peptide, 0.01-0.5 part of Oviductus Ranae small molecular peptide, 0.05-0.5 part of royal jelly protein peptide, 0.2-1.0 part of collagen peptide, 2-20 parts of biological polysaccharide, 2-20 parts of a transdermal enhancer, 5-30 parts of an emulsifier, 0.5-30 parts of a humectants, and 50-80 parts of water. By using the small molecular peptide and plant essence, and with the help of the transdermal enhancer, transdermal absorption of functional substances can be promoted, thus reaching the purposes of breast enhancement and beautification.
A kind of beverage containing ginseng and wood frog oil and its preparation method
InactiveCN102283415AMilky whiteGive full play to the health functionFood preparationGinsengOviductus Ranae
The invention provides a health-care beverage containing ginseng and oviductus ranae, which comprises the following components in percentage by weight: 0.01-0.1 percent of ginseng, 0.01-0.1 percent of oviductus ranae, 0.1-0.3 percent of tuckahoe and 0.1-0.5 percent of lotus seed. The health-care beverage disclosed by the invention has health-care efficacies of improving the immunity and resistingageing of a human body if the health-care beverage is used for a long term. The invention further provides a preparation method of the health-care beverage.
Owner:孙洪斌 +1
Oviductus ranae microcapsules and preparation method thereof
InactiveCN105533750ARetain kidney and essenceIt has the function of nourishing kidney and benefiting essenceFood shapingAcetic acidChloride
The invention discloses oviductus ranae microcapsules and a preparation method thereof, and belongs to the technical field of processing of functional oil and fat products. The microcapsules are prepared with sodium alginate and chitosan serving as wall materials and oviductus ranae serving as a core material. The microcapsules are prepared from, by mass, 1-3% of sodium alginate, 1-3% of chitosan, 1.5-8% of oviductus ranae, 2-6% of anhydrous calcium chloride, 0.5-1% of tween-80, 1% of acetic acid and the balance water. The preparation method of the microcapsules includes the main steps that 1, a wall material solution and an immobilization solution are prepared; 2, oviductus ranae and a sodium alginate solution are mixed according a certain proportion; 3, emulsification homogenization treatment is carried out; 4, the microcapsules are prepared through a piercing-solidifying method. According to the microcapsules and the preparation method thereof, bioactive substances of oviductus ranae are maintained, and many defects of oviductus ranae are overcome. The microcapsules are convenient to preserve, and the fishy smell of oviductus ranae is removed. The preparation method is easy to implement and beneficial for industrial production and opens up a new field for deep processing of rana temporaria.
Owner:JILIN UNIV
Preparation method for instant oviductus ranae
The invention provides a preparation method for instant oviductus ranae. The preparation method comprises the following steps of (1) removing impurities from the oviductus ranae to be used; (2) soaking the oviductus ranae completely, loading into a mould with 54 mm * 54 mm * 22 mm or 68 mm * 50 mm * 12 mm, then sending into a lyophilized bin, quick-freezing for 30-40 minutes at a temperature of from -30 DEG C to -38 DEG C, drying for 30-40 minutes in a vacuum environment with an absolute pressure of 1torr, at a temperature of 30-38 DEG C; (3) breaking the vacuum with clean and dry air or nitrogen after freezing and dying operations in the step (2) are finished, unloading materials after air pressure in the lyophilized bin balances with external atmospheric pressure, and storing. The oviductus ranae prepared by the method has more diversified nutritional structure, and is convenient for eating, carrying and storing.
Owner:于晶
Traditional Chinese medicine mattress for enhancing health and promoting sleep
InactiveCN104799616AImprove immunityPromote blood circulationAmphibian material medical ingredientsHeavy metal active ingredientsMonkshoodsSide effect
The invention discloses a traditional Chinese medicine mattress for enhancing health and promoting sleep, wherein the inner layer of a mattress is filled with a dry powder-shaped Chinese medicinal material. The Chinese medicinal material is prepared by mixing the following medicinal materials in parts by weight: 15 parts of bunge auriculate root, 15 parts of zinnober, 10 parts of albizia flower, 12 parts of radix ophiopogonis, 15 parts of semen ziziphi spinosae, 30 parts of tuber fleeceflower stem, 15 parts of camel fat, 9 parts of fennelflower, 12 parts of myrobalan, 9 parts of poria, 9 parts of fushen, 9 parts of liquorice, 6 parts of pine leaf, 7 parts of fluorite, 8 parts of red rhodobryum, 15 parts of monkshood and 5 parts of oviductus ranae. Clinical experiments prove that the traditional Chinese medicine mattress is excellent in effect for enhancing health and promoting sleep, safe, free from toxic and side effects and quite excellent in curative effect by virtue of selecting the appropriate medicinal materials and matching.
Owner:DONGGUAN WSDAY BEDDING TECH
Gel-like oviductus ranae health-care food and preparation method thereof
InactiveCN102511799APreserve featuresReduce churnFood preparationChinese charactersCoronary heart disease
The invention relates to a gel-like oviductus ranae health-care food. The health-care food comprises oviductus ranae and jelly composited with the oviductus ranae. The jelly is prepared from colla corii asini or tortoise-plastron glue or gelatin or mixture of the colla corii asini, the tortoise-plastron glue and the gelatin. The health-care food has the functions of delaying senility, resisting fatigue, tonifying the kidney to arrest spontaneous emission, moistening the lung and nourishing yin (Chinese character), and regulating and improving coronary heart diseases, and has good taste and appearance shape.
Owner:高耸 +1
Nutritional ointment for treating foot and hand cracks
InactiveCN102309749APromote circulationPromote healingAmphibian material medical ingredientsOrganic active ingredientsCuticleTherapeutic effect
The invention discloses a nutritional ointment for treating foot and hand cracks, which comprises the following components: oviductus ranae, collagen, apricot kernel oil, olive oil, three-dimensional copeptin, hyaluronic acid, ginseng and vaseline. The nutritional ointment has the effects of promoting blood circulation, improving metabolism, nourishing skin, softening and repairing dry skin and promoting the cracks to heal and is easy to absorb. The cracks are cleaned, the nutritional ointment is coated onto the cracks, and the cracks are slightly massaged. The nutritional ointment has a very good treatment effect on both skin epiderm cracks and chapped cracks and also can be used for along time to nourish skin.
Owner:施建军
Drug for curing porcine contagious pleuropneumonia and preparation method thereof
ActiveCN103656315AGood curative effectQuick resultsAntibacterial agentsAmphibian material medical ingredientsSide effectStephania
The invention discloses a drug for curing contagious pleuropneumonia and a preparation method thereof. The drug comprises the following raw crude drugs: magnolia bark, vernonia cinerea, oviductus ranae, peppergrass, scutellaria root, goldthread root, humble plant, conyzae herba, rhodiola root, bamboo shavings, rice distillate, common reed rhizome, semen raphani, Japanese raspberry root, mezoneuron, small fruit fig aerial root, head stephania root, lycoperdales, castanea seguinii and thermopsis lanceolata. The drug has the benefits that the drug takes heat clearing, fire purging, antisepsis, anti-inflammation, lung nourishing, yin nourishing, and cough and asthma relieving as therapeutic principles, has a good curative effect on curing contagious pleuropneumonia, is quick to become effective, has small toxic and side effects, and is low in cost.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Facial cleanser capable of promoting wound healing
InactiveCN104306212ATo promote metabolismPromote healingCosmetic preparationsToilet preparationsSide effectAnimal extract
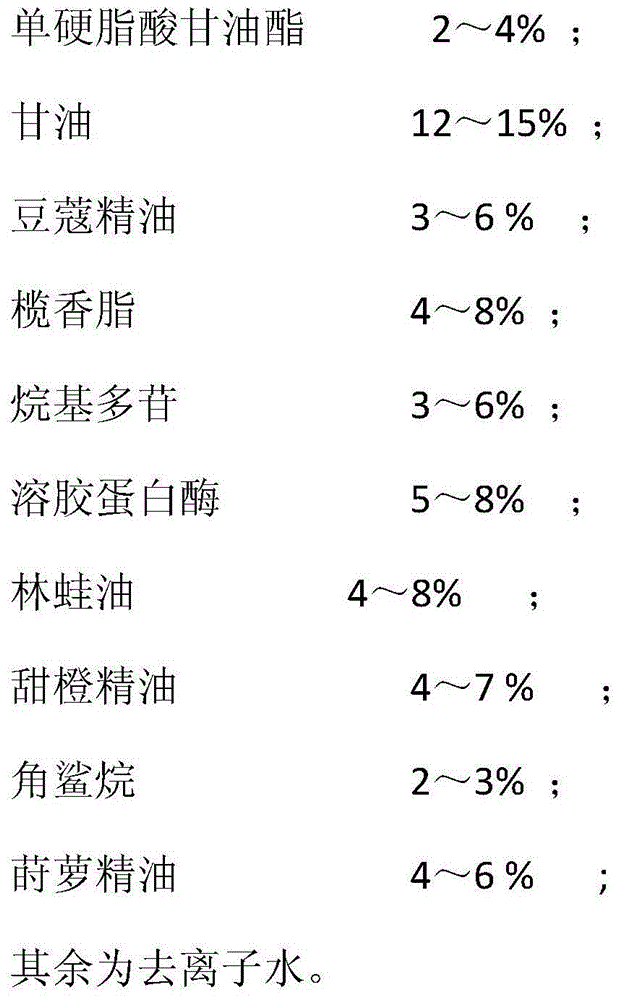
The invention relates to daily necessities, in particular to a facial cleanser capable of promoting wound healing, which mainly comprises glycerol monostearate, glycerol, cardamom oil, elemi, alkyl glucosides, sol protease, oviductus ranae, sweet orange oil, squalane, dill oil and deionized water. The facial cleanser promotes the wound healing when promoting the metabolism of body and relieving pain, and heals wounds, contains natural plant and animal extracts without any side effects, and is safe to use.
Owner:QINGDAO KANGHEWEIYE COMML
Medicinal composition for treating vaginal dryness
InactiveCN102688260ASimple recipeEasy to produceAmphibian material medical ingredientsSexual disorderDuring menopauseOvarian function
The invention discloses a medicinal composition for treating vaginal dryness. The composition comprises 50-80 wt% of Oviductus Ranae, and 20-50 wt% of Chinese herbal powder. The medicinal composition has effects in invigorating kidney and vital essence, nourishing Yin and blood, promoting qi circulation to remove stagnation; and can be used for treating symptoms such as vaginal dryness, sexual pain and sexual hypoactivity due to perimenopause or ovary dysfunction. The composition is simple in formula and production, and effective in curative effect, is prepared from pure Chinese medicinal materials, and has no toxic and side effects.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Low-fat and sugar-free oviductus ranae health-care yoghourt and preparation method thereof
InactiveCN103238668AUniform and delicate tissue stateDelicate and smooth tasteMilk preparationSucroseAdditive ingredient
The invention discloses low-fat and sugar-free oviductus ranae health-care yoghourt and a preparation method thereof and belongs to the technical field of food processing. The method comprises the following main steps of according to fermentation base materials, inoculating 0.01 to 0.05g / L mixed directly adding fermenting agent of lactobacillus bulgaricus and Streptococcus thermophilus into slurry which contains 20 to 50 percent of fresh milk, 3 to 7 percent of dried skim milk, 8 to 14 percent of xylitol and oviductus ranae; fermenting for 4 to 10 hours at the temperature of 40 to 50 DEG C; and curing the product at low temperature to obtain the oviductus ranae health-care yoghourt. The oviductus ranae health-care yoghourt prepared by the method is rich in activated probiotics and a plurality of functional components, low in fat and free of sugar, has the effects of regulating intestinal flora balance, keeping health of intestines, reducing blood fat and blood sugar, protecting liver, resisting cancer, beautifying and the like, is particularly suitable for middle-aged and aged people and diabetics, and provides a new way for deep processing of oviductus ranae.
Owner:JILIN UNIV
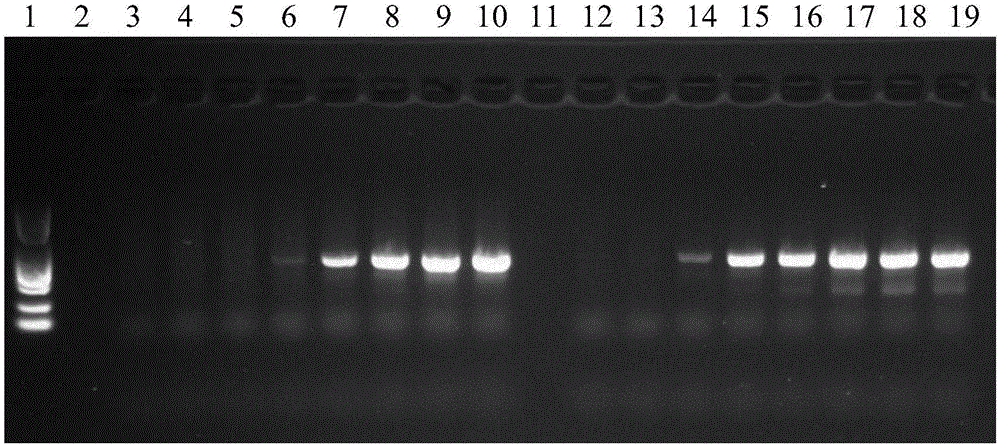
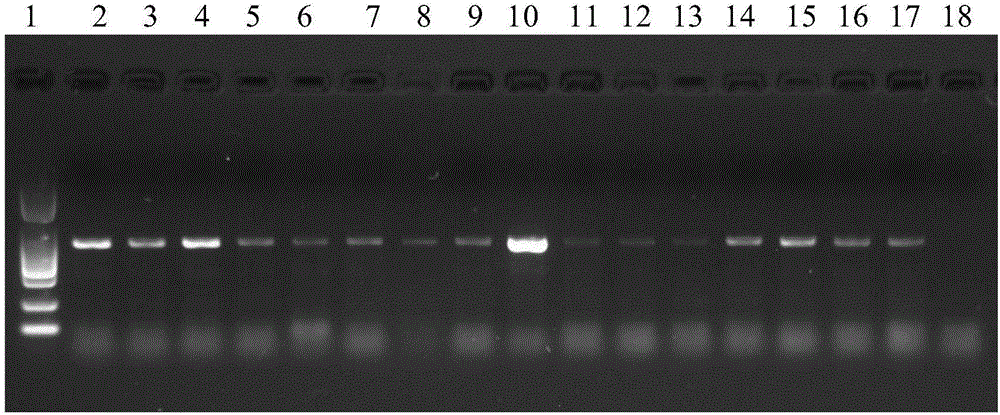
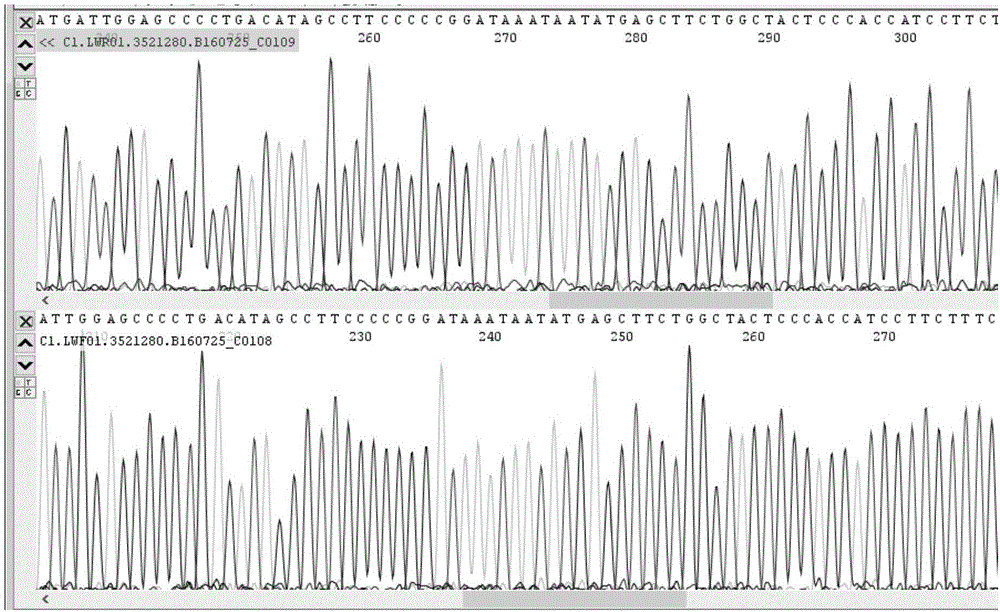
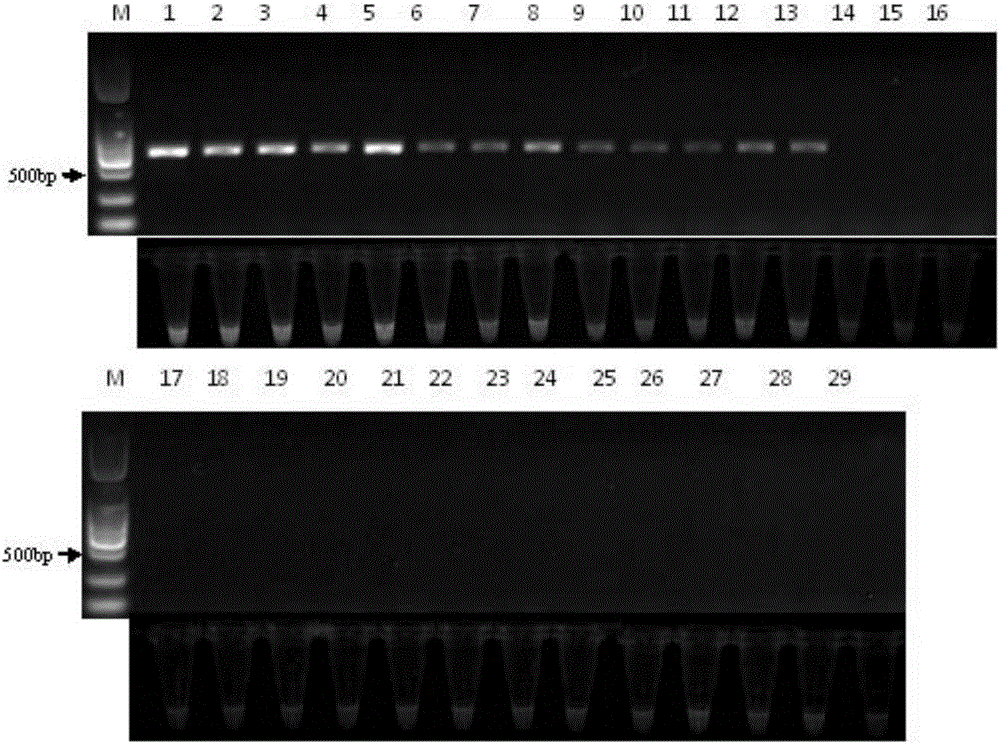
PCR (polymerase chain reaction) amplification primer for oviductus ranae medicinal material DNA (deoxyribonucleic acid) bar code identification and amplification method
ActiveCN106755452AStrong specificityEfficiently obtainedMicrobiological testing/measurementDNA/RNA fragmentationForward primerAgricultural science
The invention discloses a PCR (polymerase chain reaction) amplification primer for oviductus ranae medicinal material DNA (deoxyribonucleic acid) bar code identification. A forward primer sequence of a primer pair is as shown in SEQ ID No.1, and a reverse primer sequence is as shown in SEQ ID No.2. The invention further provides an amplification method for obtaining an oviductus ranae medicinal material DNA bar code. PCR amplification is performed through primers as shown in SEQ ID No.1 and shown in SEQ ID No.2, and the conditions of PCR amplification are as follows: denaturation is performed for 1 minute at 94 DEG C; denaturation is performed for 1 minute at 94 DEG C, annealing is performed for 1.5 minute at 50 to 54 DEG C, extending is performed for 1 minute at 72 DEG C, and 35 cycles are preformed; extending is performed for 5 minutes at 72 DEG C. The PCR amplification primer and the amplification method have the advantages that the PCR specific amplification is high, the oviductus ranae medicinal material DNA bar code can be rapidly and effectively obtained, and performing oviductus ranae medicinal material and original animal identification through a DNA bar code technology becomes possible.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI +1
External traditional Chinese medicine for treating allergic rhinitis
InactiveCN104491806APromote and adjust immune functionPromotes and regulates disease resistanceAmphibian material medical ingredientsHeavy metal active ingredientsHouttuyniaPolygala tenuifolia
The invention provides external traditional Chinese medicine for treating allergic rhinitis. The external traditional Chinese medicine is characterized in that the following traditional Chinese medicine raw materials are prepared into ointment according to the following preparation process in weight ratio: 10g of fresh ginger, 10g of fresh houttuynia cordata, 10g of fresh mint leaf, 50g of cicada slough, 50g of vinegar rhizoma corydalis, 50g of peach kernel, 50g of concha arcae, 50g of myrobalan, 50g of calamine, 50g of red halloysite, 75g of radix angelicae, 25g of fructus cnidii, 75g of clove, 10g of resina draconis, 20g of frankincense, 20g of myrrh, 50g of cocklebur fruit, 30g of magnolia flower, 5g of asarum, 50g of rhizoma zingiberis, 25g of ephedra, 50g of cassia twig, 50g of yam, 50g of sealwort, 50g of fructus lycii, 25g of oviductus ranae, 50g of lotus seed meat, 50g of raspberry, 50g of notopterygium root, 30g of ligusticum wallichii, 50g of folium artemisiae argyi, 50g of orange peel, 50g of perilla leaf, 10g of rhizoma curculiginis, 50g of codonopsis pilosula, 15g of licorice, 60g of loofah sponge, 50g of Chinese starjasmine stem, 50g of dioscorea nipponica makino, 40g of polygala tenuifolia, 50g of cortex albiziae and 50g of ligusticum sinense oliver. The external traditional Chinese medicine provided by the invention treats both symptoms and root causes, and the herbs collectively act to perfect the somatic functions on the whole, so as to cure diseases.
Owner:张景悦
Soft capsule containing oviductus ranae
InactiveCN103520239AAvoid side effectsGood curative effectAmphibian material medical ingredientsDigestive systemVegetable oilMedicine
The invention relates to a soft capsule containing oviductus ranae. The soft capsule comprises content matters and a capsule shell, wherein the content matters contain the following materials in percentage by mass: 10-50% of oviductus ranae meal, 3-30% of ginseng extract, and 20-80% of vegetable oil. The soft capsule is prepared from the ginseng extract and oviductus ranae meal, active ingredients in the ginseng extract and oviductus ranae meal can supplement with each other, namely can be complementary, the oviductus ranae has the effects of tonifying the kidney and nourishing the essence, and nourishing the lung and yin, and ginseng has functions of treating collapse and cardiovascular diseases as well as stomach and liver diseases, so that the soft capsule has the function of tonifying the internal organs of the body in general and is capable of enhancing the human organism immunity and prolonging the service life of the organism after being eaten.
Owner:关成军
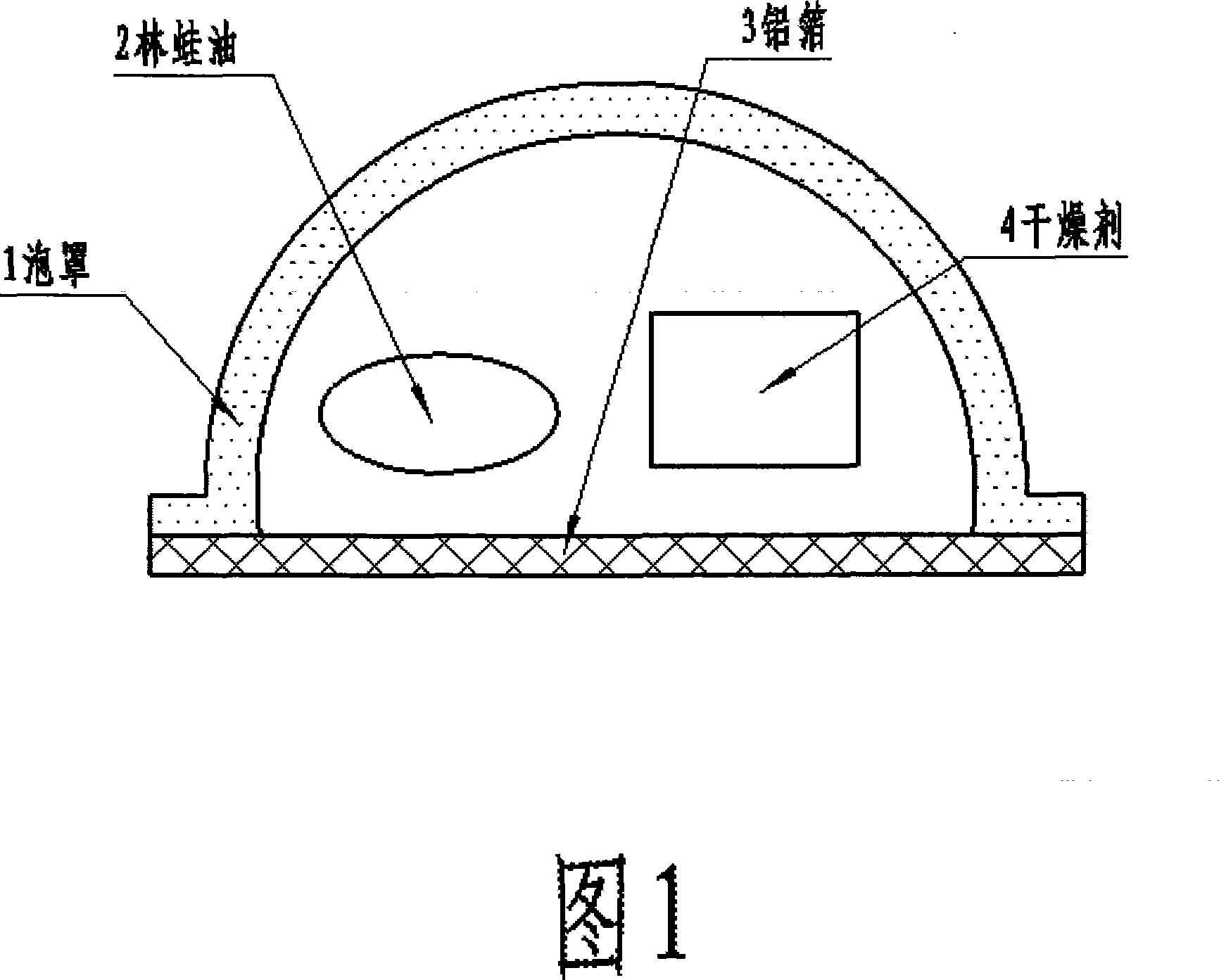
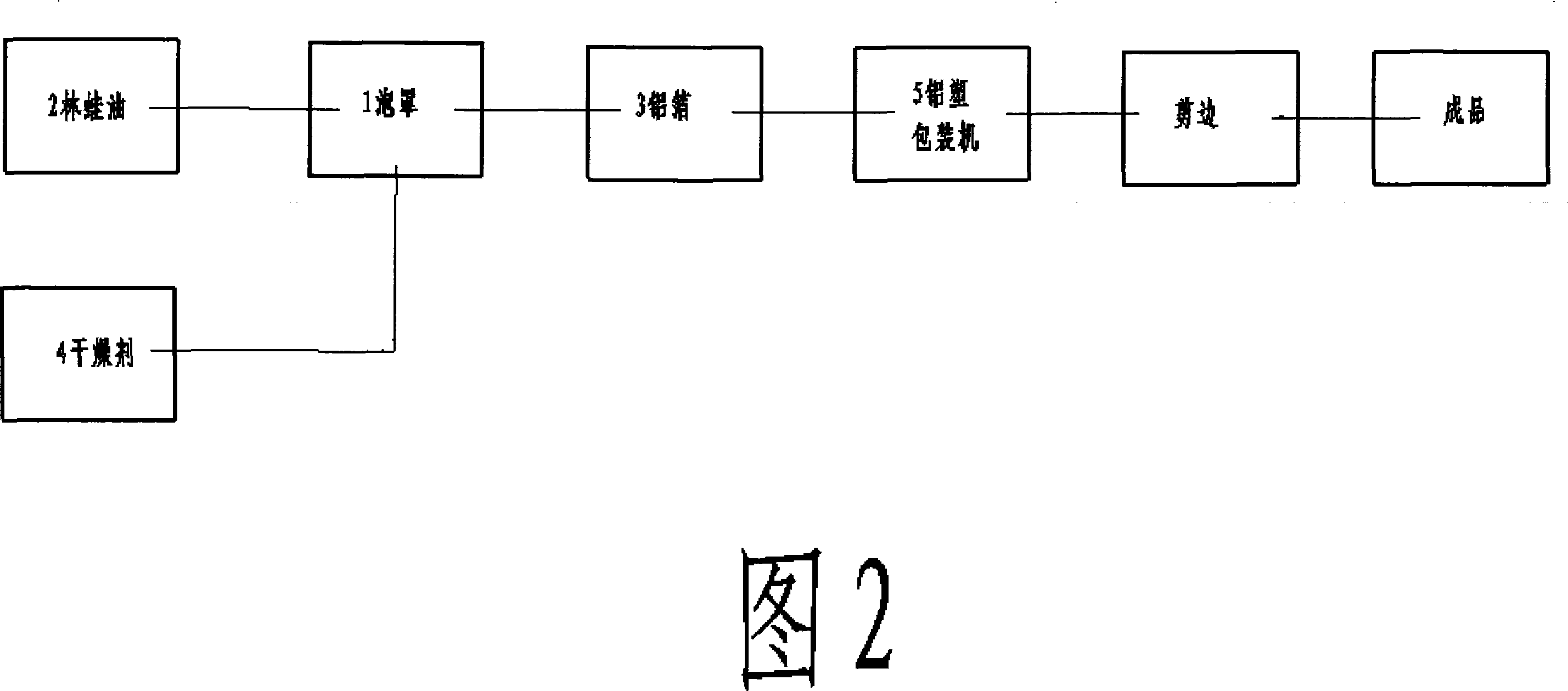
Aluminium-plastic sealing method for wood-frog oil
InactiveCN101070111AThe quality remains the sameSimple structureContainers preventing decayDesiccantOviductus Ranae
The invention discloses a new method to seal oviductus ranae using the aluminium plastic, which prevents the bad influence to it from the change of the climate and humidity and avoids the oviductus ranae mildew. The method is to put the dried oviductus ranae and the dryer into the bubble cap which forms the sealing room with the aluminium plastic after compressing by the aluminium plastic packing machine. Also it is sealed alone, so it will not influence each other in eating process.
Owner:李英鹏 +2
Oviductus ranae soft capsule and manufacturing process thereof
ActiveCN101642472ADoes not destroy nutrientsImprove biological activityAmphibian material medical ingredientsDigestive systemOviductus RanaeTwo step
The invention relates to a manufacturing process of oviductus ranae soft capsules, which adopts the technical scheme that the oviductus ranae soft capsule is prepared from infused oviductus ranae, soybean salad oil, vitamin E and glycerin monostearate; the manufacturing process comprises the following steps: taking fresh oviductus ranae, microwave sterilizing for 2-3 minutes at the temperature of30-50 DEG C after washing, sending into a freeze drier, and freezing for 7-9 hours at the temperature of minus 45-minus 35 DEG C; staring a vacuum pump, and heating and sublimating for 9-11 hours at the temperature of 25-35 DEG C and under the vacuum degree of 1.3-13 Pa; heating and sublimating for 11-13 hours at the temperature of 55-65 DEG C; and heating and sublimating for 9-11 hours at the temperature of 75-85 DEG C to prepare active oviductus ranae; and cooling the soybean salad oil, the vitamin E and the glycerin monostearate in two steps, emulsifying and homogenizing the soybean salad oil, the vitamin E, the glycerin monostearate and the infused oviductus ranae, and finally pelletizing through a soft capsule pelletizer. The oviductus ranae soft capsule not only has high oil content,but also can fully preserve the health components of the oviductus ranae.
Owner:BENXI LONGBAO GROUP GINSENG & VELVET
Oviductus ranae wine and preparation method thereof
InactiveCN106554891AImprove immunityAnti agingAmphibian material medical ingredientsAntinoxious agentsHigh concentrationOviductus Ranae
The invention relates to a nutritional health wine, in particular to an oviductus ranae wine and a preparation method thereof. The oviductus ranae wine is prepared from 1 part of oviductus ranae and 500-1000 parts of wine. The preparation method includes: (1) removing impurities from oviductus ranae and performing washing; (2)-(4) stirring oviductus ranae three times at 48-80DEG C for 30-40min, and performing cooling to a temperature ranging from -3DEG C to 0DEG C after each stirring, thus obtaining precipitate-free emulsus high concentration oviductus ranae wine; (5) adding the high concentration oviductus ranae wine obtained in step (4) into the residual wine; and (6) carrying out sterilizing and filling the wine obtained by step (5), thus obtaining the oviductus ranae wine. According to the method, oviductus ranae insoluble in ethanol is completely dissolved in wine to fuse with wine together completely, thus being better absorbed by the human body. The oviductus ranae wine prepared by the method provided by the invention has a nutritional component content 35-40% higher than that of oviductus ranae wine prepared by the existing method.
Owner:杨秀武
Health-care food with sleep improving function
The invention discloses health-care food with a sleep improving function. The health-care food with the sleep improving function comprises the following active components in part by weight: 10-250 parts of spina date seed, 5-200 parts of polygala tenuifolia, 5-150 parts of schisandra, 5-150 parts of platycladi seeds and 1-25 parts of oviductus ranae. The health-care food with the sleep improving function has the characteristics that: features of traditional Chinese herbal medicines are combined with modern medical research, various components are reasonably formulated, matched and used together, and through a variety of ways and a variety of levels, the sleep improving health-care function is achieved.
Owner:INFINITUS (CHINA) CO LTD
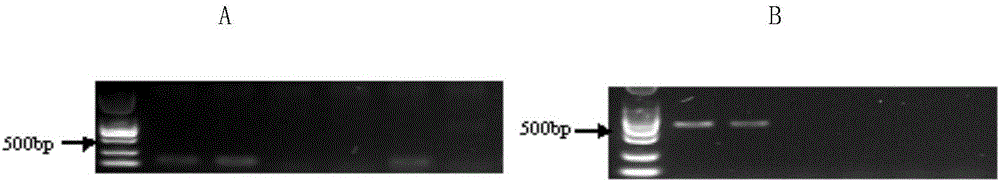
Method for performing true and false identification on oviductus ranae and special primer
The invention discloses a method for performing true and false identification on oviductus ranae and a special primer. The invention provides a primer pair for performing true and false identification on oviductus ranae provided in the invention, and the primer pair is a primer pair capable of amplifying a DNA fragment A, wherein the DNA fragment A is a product obtained by taking the oviductus ranae as a template and amplifying the template by using the primer pair shown as a sequence 1 and a sequence 2. According to the study, by optimizing conditions of locus specific PCR (polymerase chain reaction), the aim of simply, rapidly and intuitively identifying the oviductus ranae and common counterfeit species thereof within 40 minutes is achieved, and accurate and rapid field identification of oviductus ranae medicinal materials is realized.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Preparation method of oviductus ranae polypeptide
A preparation method of oviductus ranae polypeptide specifically comprises steps: (1) enzymolysis, (2) blending, (3) preparation of an emulsifier, (4), high-speed shearing, and (5) freeze drying. In the method, freeze drying for powder preparation is adopted, and an oviductus ranae enzymatic hydrolysate is taken as a raw material. After dried oviductus ranae is made into the enzymatic hydrolysate, based on the enzymatic hydrolysate, oviductus ranae enzymatic hydrolysate powder low in hygroscopicity and good in resolvent performance is prepared through freeze drying and processes of reasonable blending and emulsification.
Owner:HEILONGJIANG JOHNSUN BIOLOGICAL ENG CO LTD
Oviductus ranae preparation method and instant oviductus ranae food
InactiveCN106942718AMaintain textureImprove user experienceAnimal feeding stuffFood ingredient as taste affecting agentChemistryOviductus Ranae
The invention discloses an oviductus ranae preparation method, which comprises: pre-treating dry oviductus ranae; adding purified water at a low temperature while introducing ozone; and carrying out impregnating swelling, and dewatering to obtain the oviductus ranae. The invention further discloses a food prepared by using the method. According to the oviductus ranae prepared by using the method and the food, the problems of rotting and fishy odor due to the soaking swelling of the dry oviductus ranae through water are solved, the original structure of the oviductus ranae is maintained, and the good taste is provided.
Owner:深圳华大优选科技有限公司 +1
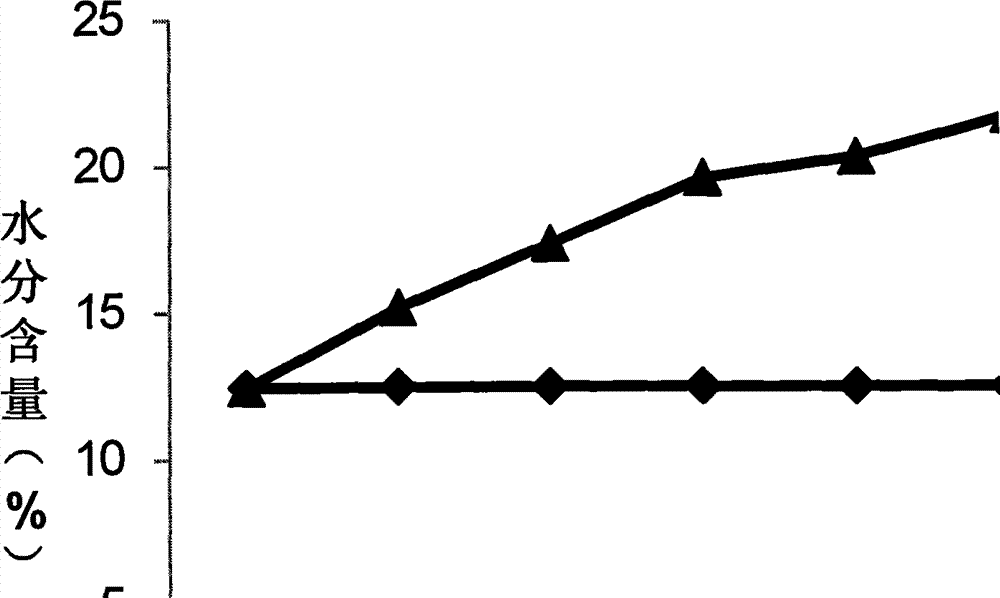
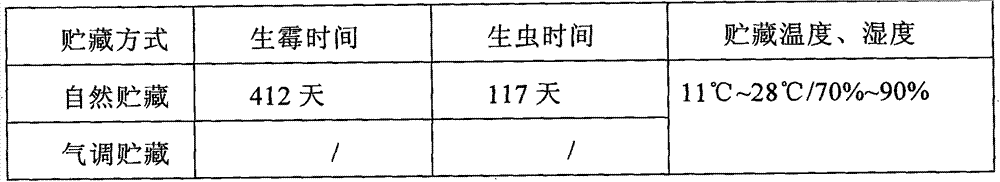
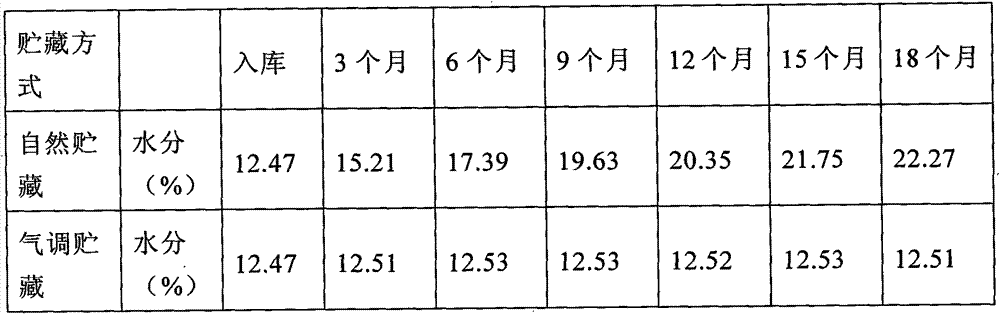
Controlled atmosphere storage process of such Chinese herbal medicines as corn cervi pantotrichum, oviductus ranae and odobenus rosmarus
InactiveCN104760765APrevent pestsExtended storage periodPackage sterilisationPackaging under vacuum/special atmosphereOviductus RanaeTraditional medicine
The invention discloses a controlled atmosphere storage process of such Chinese herbal medicines as corn cervi pantotrichum, oviductus ranae and odobenus rosmarus. The process comprises the following steps: (1) bagging: dry products of Chinese herbal medicines are loaded into controlled atmosphere bags; (2) gas distribution: the needed controlled atmosphere ratios of N2: (47-59)%, O2: (1-3)% and CO2: (40-50)% are prepared by a gas distribution machine, and are gas volume ratios; (3) inflation: the bagged Chinese herbal medicines are inflated by a controlled atmosphere packing machine; (4) packing: the inflated Chinese herbal medicines are packed by an aluminum nail sealing machine; (5) disinfection: the dry products of the Chinese herbal medicines loaded into the controlled atmosphere bags are ultravioletly disinfected, and are irradiated for 4-5 min by a 20 W ultraviolet lamp according the distance of 1+- 0.1 m; and (6) warehousing: the disinfected packed products are conveyed into a dry warehouse for storage. The controlled atmosphere storage process can prolong the storage period of the Chinese herbal medicines, prevents the loss of effective components due to the damp, the mould and the volatility, and prevents the Chinese herbal medicines from being damaged by insects.
Owner:邓彬
Instant oviductus ranae and preparation method thereof
The invention provides instant oviductus ranae, which takes oviductus ranae as raw materials, and is prepared through a method as follows: purifying and deodorizing the oviductus ranae, curing at high temperature, sterilizing, freezing, vacuum drying, and preparing to be instant oviductus ranae food. The instant oviductus ranae is convenient to eat, is easy to store, is convenient to carry, has no additive, and is safe to eat. The invention also provides a preparation method of the instant oviductus ranae. The method is simple in process without using food additives.
Owner:桦甸市吉元土产有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com