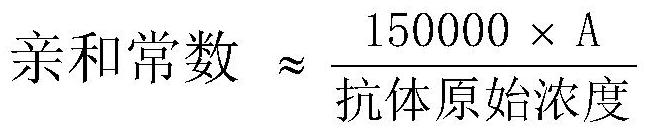Anti-CD5 protein monoclonal antibody as well as cell strain, preparation method and application thereof
A monoclonal antibody and protein technology, applied in the field of biomedical engineering, can solve problems such as unclear mechanism and achieve high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
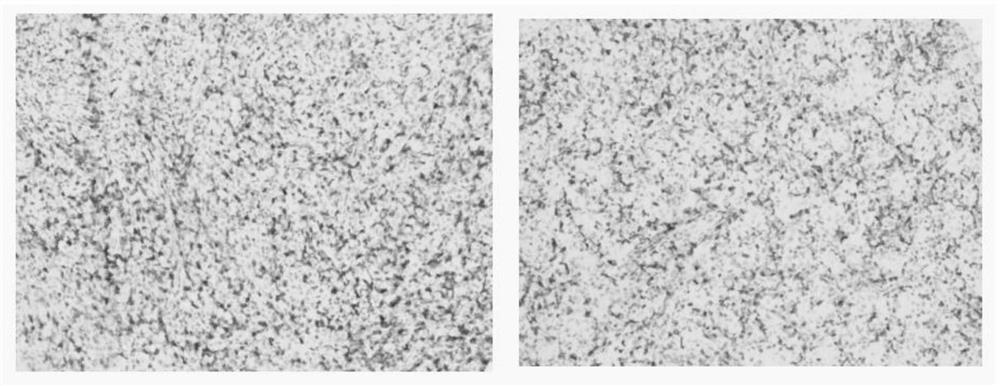
Embodiment 1
[0018] The preparation of embodiment 1 recombinant CD5 protein fragment
[0019] 1. Gene optimization and synthesis
[0020] According to the protein sequence with accession number O43866 in the Uniprot database, CD5 selects the protein fragment at positions 398-495, which is the amino acid sequence shown in SEQ ID NO.1; it is directly optimized into a gene fragment suitable for expression in Escherichia coli BL21. During the PCR process, BamH I and XhoI restriction sites were added to the 5' and 3' ends of the gene, respectively.
[0021] The PCR products were separated by agarose gel electrophoresis and recovered. The recovered fusion protein gene and the plasmid vector Pet30a used for expression were digested with BamHI and XhoI respectively, recovered by electrophoresis again, and ligated with T4 DNA ligase. The ligation product was transformed into Escherichia coli competent cell BL21, and the clones on the plate were picked and inoculated, and the PCR identification of ...
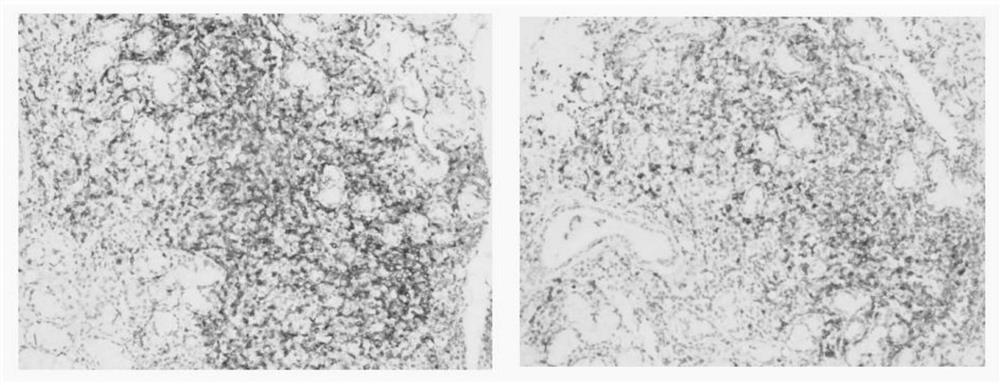
Embodiment 2
[0025] The establishment of embodiment 2 hybridoma cell lines
[0026] 1. Immunity
[0027] The recombinant protein in Example 1 was emulsified with complete Freund's adjuvant (Sigma, F5881), immunized 4-6 weeks old SPF female mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), and injected subcutaneously into the abdomen 6 points per mouse, the dose is 60 μg / mouse. Immunization was boosted every 14 days, and the antigen was emulsified with Freund's incomplete adjuvant (Sigma, F5506) at a dose of 20 μg per mouse. 7 days after the third booster immunization, indirect ELISA (wavelength 450nm) was used to detect the polyantibody titer of the anti-immunogen in the mouse serum. 20μg / only.
[0028] 2. Cell Fusion
[0029] Aseptically prepare the mouse splenocyte suspension that reaches the immune standard, mix it with mouse myeloma cell sp2 / 0 (ATCCNumberCRL-8287) at a ratio of 5:1, and centrifuge at 1500rpm for 5min. After the supernatant was d...
Embodiment 3
[0032] Example 3 Preparation of Monoclonal Antibody by Ascites Induction Method
[0033] 1. Ascites preparation
[0034] The cells in the logarithmic growth phase were washed with serum-free medium and suspended, counted about 5×10 5 , 1ml. The suspended cells were injected intraperitoneally into mice previously sensitized with paraffin oil. Ascites collection was started 7 days later. The removed ascites was centrifuged at 4000rpm for 10min at 4°C. Carefully suck out the ascites in the middle and collect in a centrifuge tube, and store at 4°C or -20°C.
[0035]2. Purification of monoclonal antibodies
[0036] Antibody was purified from ascitic fluid by HiTrap rProtein A FF (GE Company) affinity chromatography according to the instructions. The purity was identified by SDS-PAGE gel, and the concentration was determined by Bradford method. Purified antibodies were stored at -20°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com