CD5 chimeric antigen receptor for adoptive T cell therapy
a t cell and chimeric antigen technology, applied in the field of immunology, cell biology, molecular biology, medicine, can solve the problems of high risk of incurable relapse, low cure rate of adult t-all cases, and difficulty in using car t cells against t-cell malignancies, so as to improve t cell persistence and antitumor responses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CD5 CAR for T-Cell Malignancies
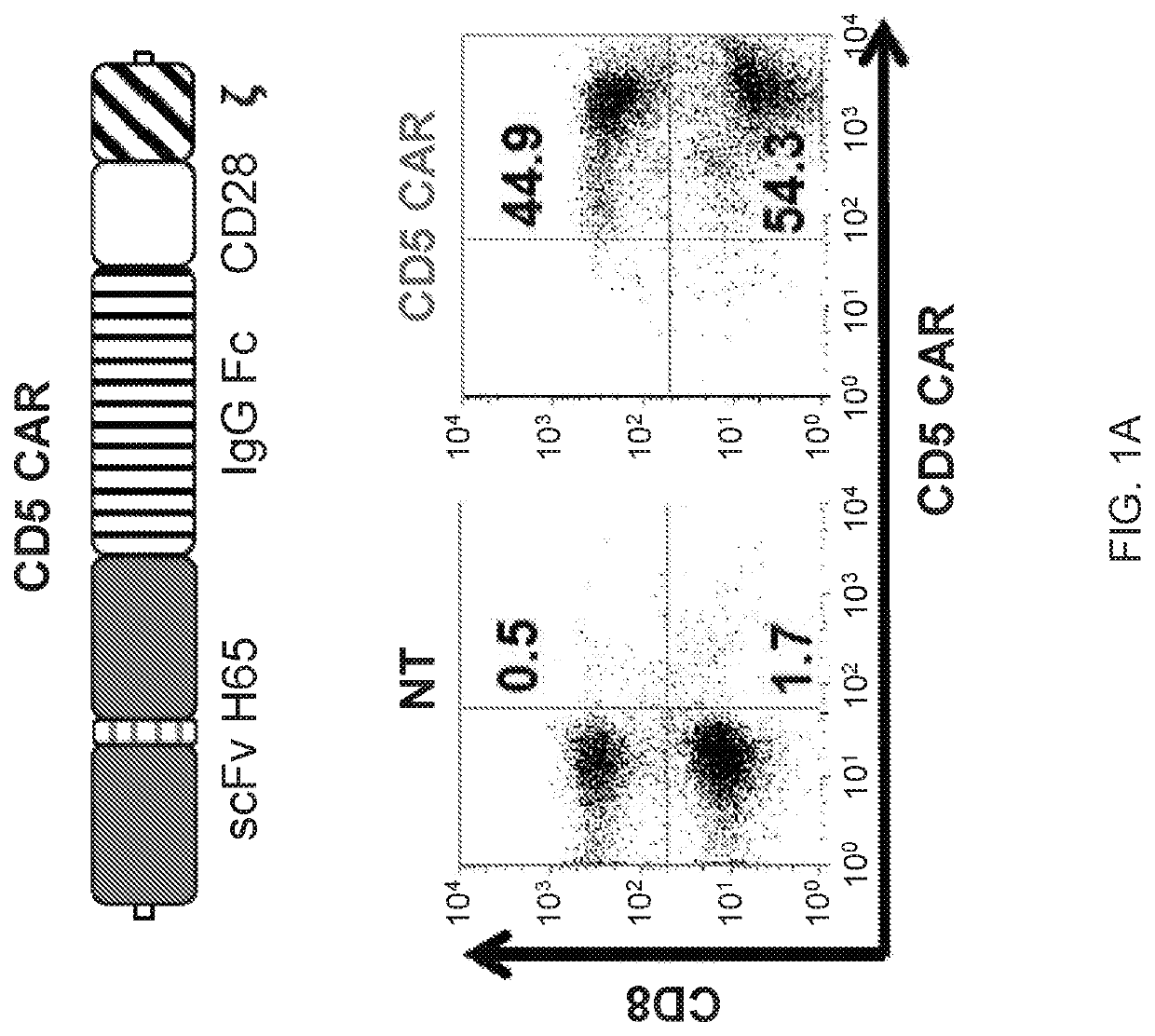
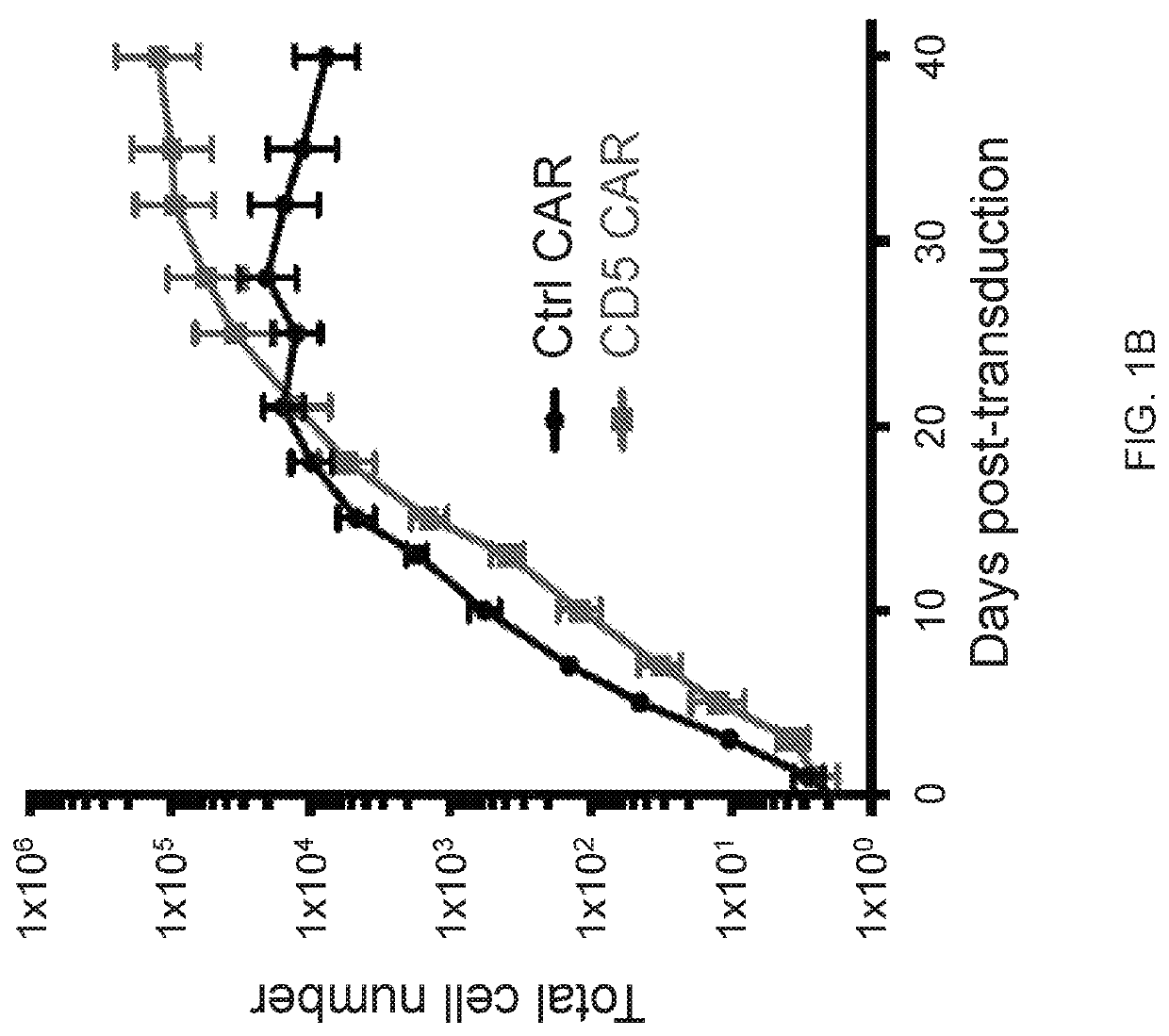
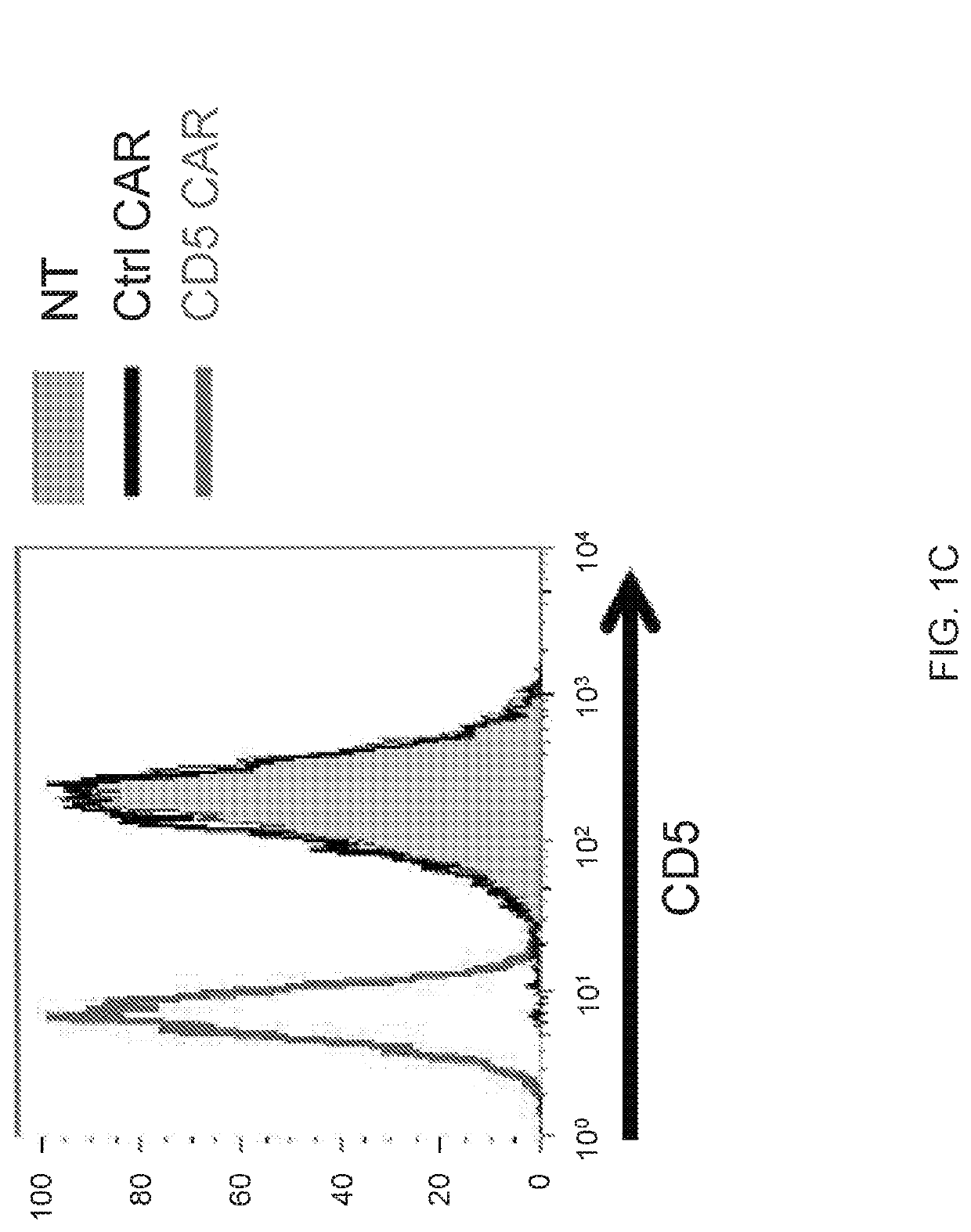
[0183]Chimeric antigen receptors (CARs) have emerged as a powerful therapeutic tool redirecting patient's own T cells to treat hematologic malignancies—in particular, those of B cell origin. However, T cell malignancies remain a more challenging task for CAR T cells owing to the shared expression of targetable tumor-associated antigens, e.g., CD5, between normal and cancerous T cells, potentially invoking fratricide of CAR T cells.
[0184]The present disclosure provides a novel CAR targeting CD5, a common marker expressed in most T-cell leukemia / lymphoma blasts and normal T cells. As described below, upon transduction with a CD5 CAR, T cells displayed limited and transient fratricide and eventually acquired resistance to self-killing. Expansion of CD5 CAR T cells coincided with downregulation of CD5 from the cell surface. At the same time, CD5 CAR T cells efficiently recognized and completely eliminated CD5-positive T-ALL and T cell lymphoma cell lines i...
example 2
A T Cell-Directed Chimeric Antigen Receptor for the Selective Treatment of T Cell Malignancies
[0185]Lymphoid malignancies produce significant morbidity and mortality in pediatric and adult patients (Dores, et al., 2012). Although recent advances in chemotherapy have improved disease-free survival, prognosis remains poor for primary chemotherapy-refractory or relapsed patients, and all patients may have significant short-term and long-term toxicities from their treatment (Kantarjian, et al., 2010; Gökbuget, et al., 2012; DeAngelo, et al., 2007; Goldberg, et al., 2003; Oudot, et al., 2008; O'Brien, et al., 2008). Recent studies in patients with B lymphoid malignancies have demonstrated the remarkable potency of chimeric antigen receptors (CARs) that can redirect T cells to the CD19 antigen present on normal and malignant B cells with complete response rates of >90% even in patients with refractory or relapsed disease (Brentjens, et al., 2013; Maude, et al., 2014). Such response rates,...
example 3
Modifications of CD5 Chimeric Antigen Receptors
[0232]In particular embodiments, a CD5 CAR has modifications to one or more components of the polynucleotide encoding the CAR and / or the immune cell harboring the polynucleotide. In certain embodiments, a particular anti-CD5 scFv is utilized, including those that are not specifically listed elsewhere herein. For the intracellular signaling domains, any suitable combination may be employed, and the skilled artisan knows how to test the efficacy and safety of the combinations based on routine practices described elsewhere herein.
[0233]One CAR component that may be varied depending on the particular CD5 CAR is the spacer and / or hinge region. A spacer region links the antigen binding domain to the transmembrane domain. In specific embodiments the spacer is flexible enough to allow the antigen binding domain to orient in different directions to facilitate antigen recognition. In certain embodiments, the hinge region is from IgG1. In specific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com