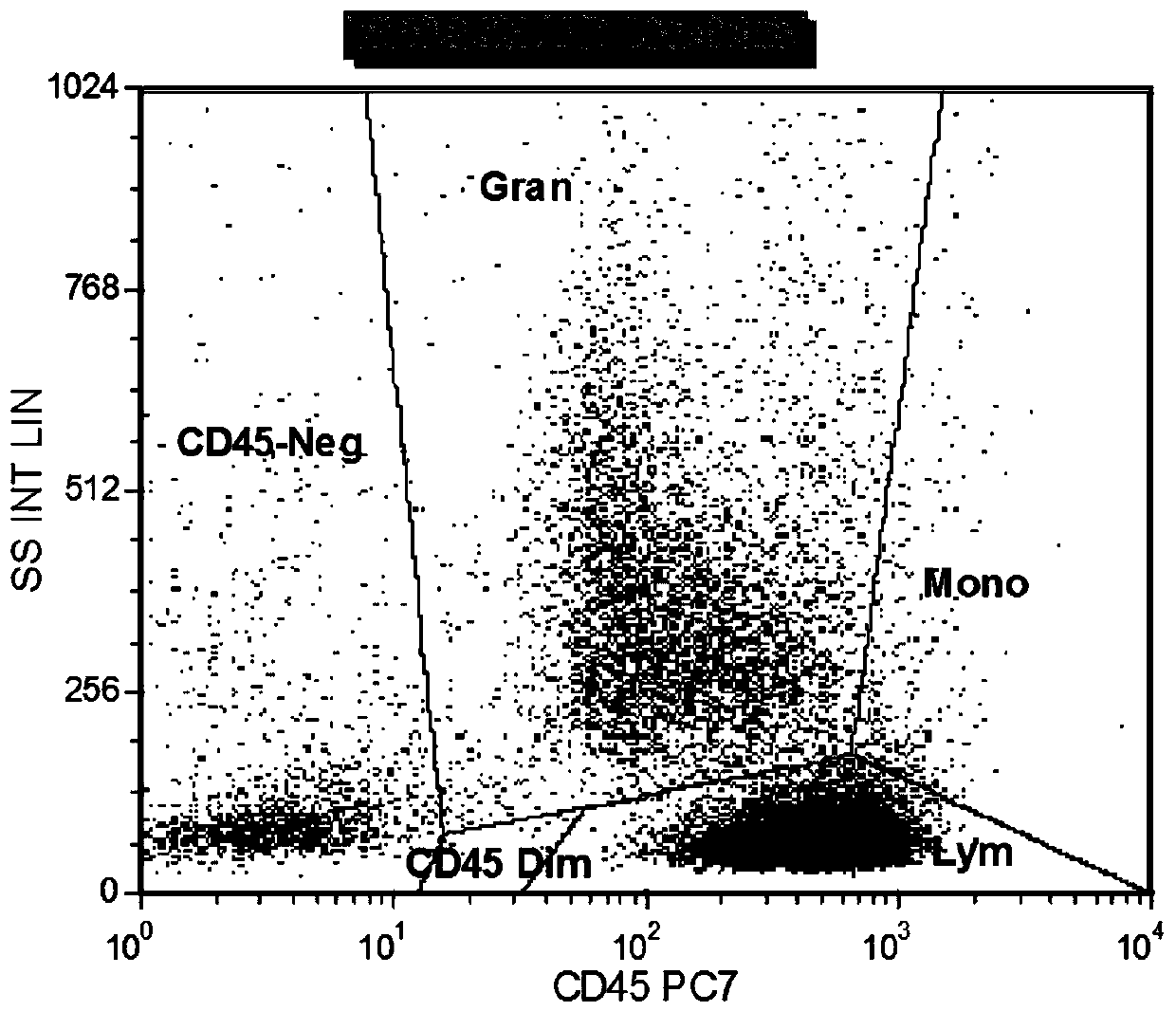
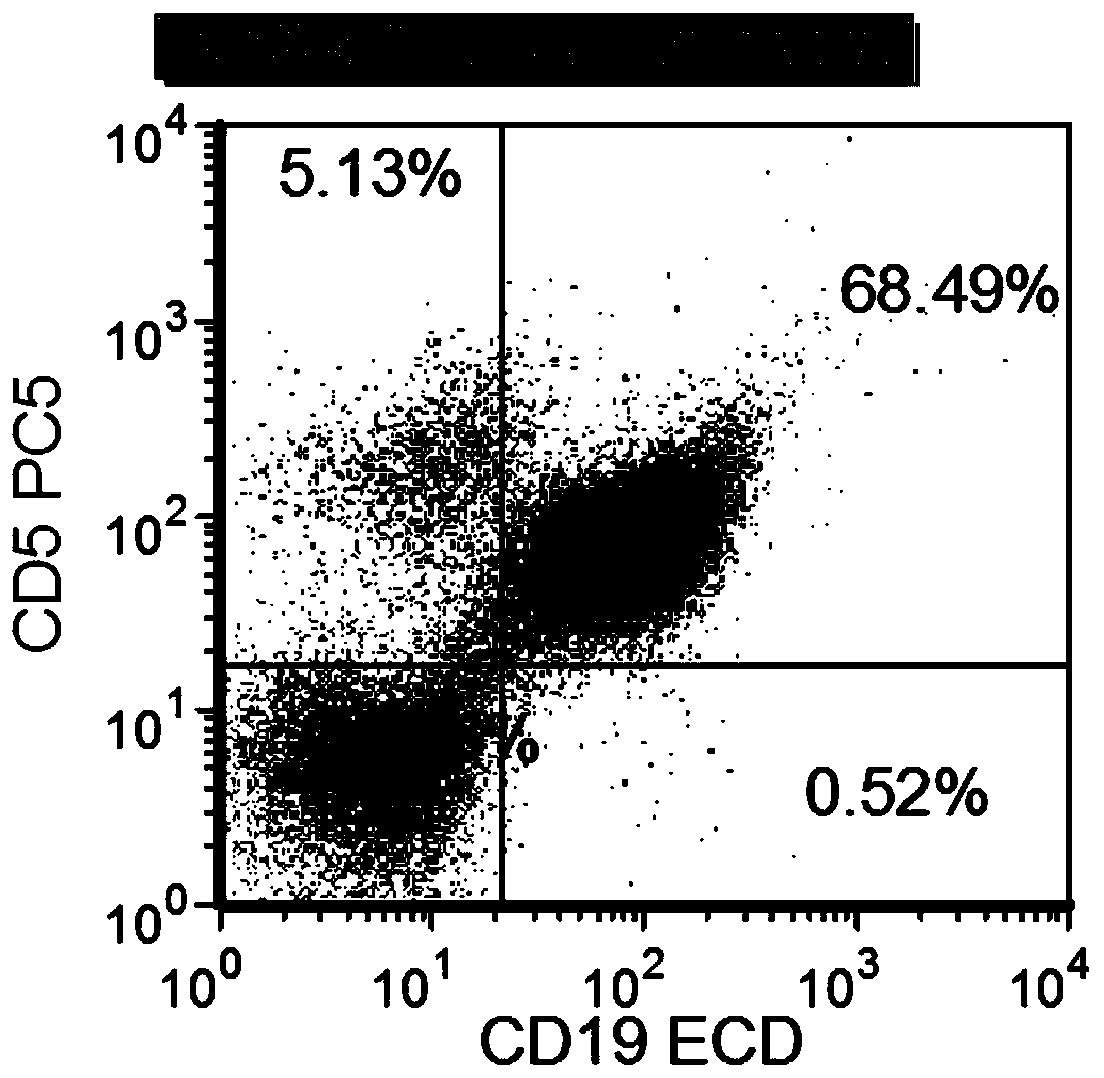
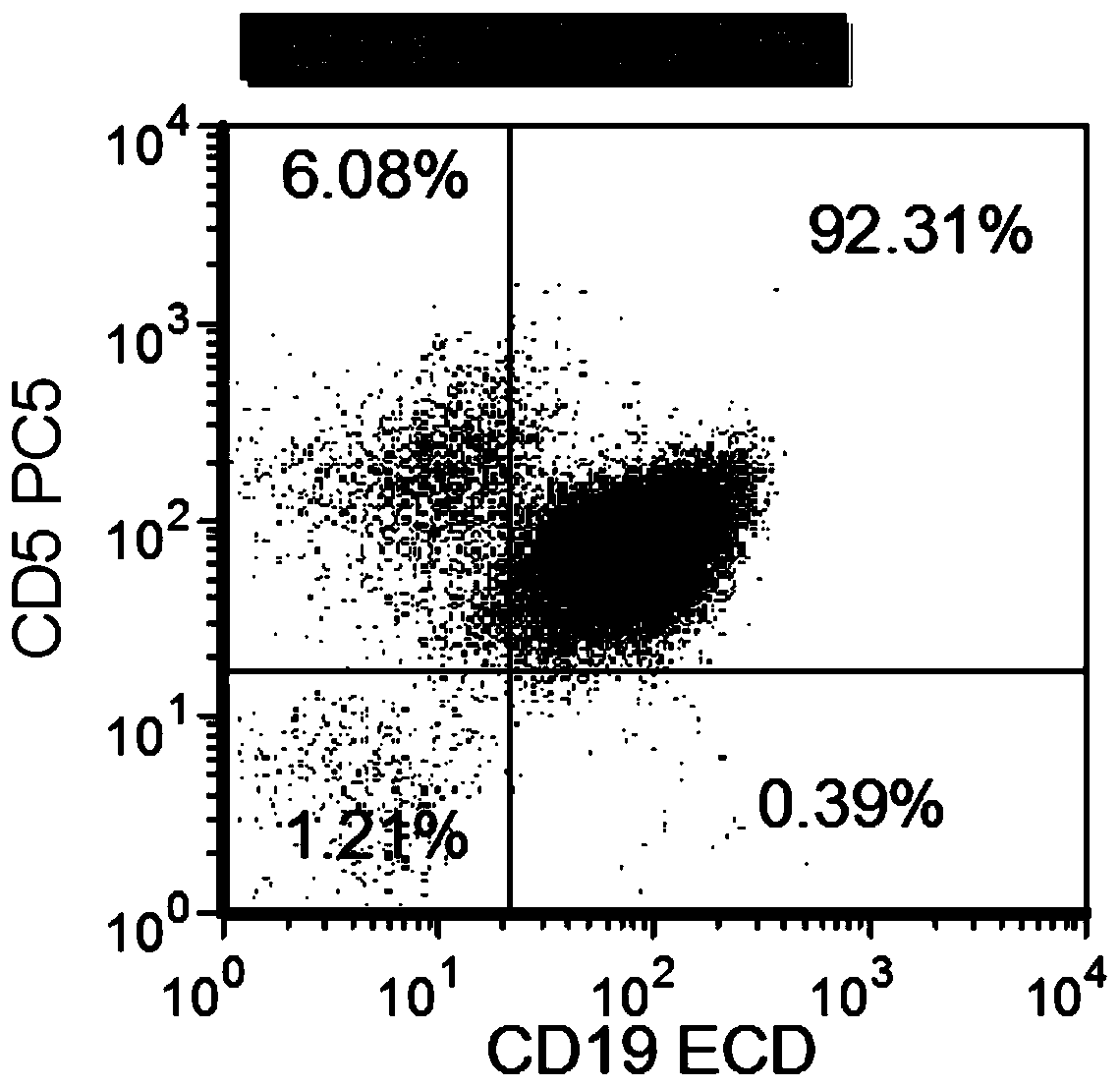
Combined reagent and system for evaluating chronic lymphocytic leukemia prognosis
A lymphocyte and leukemia technology, applied in the field of medical technology detection, can solve the problems of time-consuming and difficult to use, and achieve the effect of covering a wide range, facilitating result judgment and rapid detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A combined reagent for evaluating the prognosis of chronic lymphocytic leukemia, the monoclonal antibodies in each group are respectively labeled with the following fluorescent labels FITC, PE, ECD, PE-Cy TM 5. PE-CY7, as follows:
[0062] Antibody combination 1: CD5, IgG1, CD3, CD19, CD45;
[0063] Antibody combination 2: CD5, ZAP70, CD3, CD19, CD45;
[0064] Antibody combination 3: CD38, CD49d, CD19, CD5, CD45.
[0065] The fluorescent labels and dosages of relevant monoclonal antibodies in the above combinations are shown in the table below.
[0066] Table 2. The dosage of each monoclonal antibody
[0067] fluorescent label
[0068] Note: The concentration gradient of the commercially available antibodies was verified to determine the optimal dosage, so the above-mentioned monoclonal antibodies were loaded into flow tubes numbered 1, 2, and 3, respectively.
Embodiment 2
[0070] A system for evaluating the prognosis of chronic lymphocytic leukemia, including: a detection module, a data acquisition module and a data analysis module.
[0071] The detection module performs flow cytometry detection on the cells to be tested;
[0072] The data acquisition module acquires the flow cytometry detection result data of the cells to be tested stained with the combined reagent described in Example 1;
[0073] The data analysis module analyzes the above acquired data, and evaluates the prognosis of chronic lymphocytic leukemia according to predetermined criteria.
[0074] The specific workflow of using the above system is as follows:
[0075] 1. Prepare reagents.
[0076] The combination reagents described in Example 1 were prepared.
[0077] 2. Prepare a single cell suspension.
[0078] The sample source of the cells to be tested can be bone marrow, peripheral blood, pleural effusion, ascites, etc., and the concentration is adjusted to 1×10 according t...
Embodiment 3
[0102] The system for evaluating the prognosis of chronic lymphocytic leukemia in Example 2 was used to evaluate the prognosis of chronic lymphocytic leukemia, and 166 daily samples were randomly selected for detection. The positive rate of IGHV gene mutation detection was 81.2%, and the coincidence rate between the results of this method and the results of IGHV reached 85.3 %, proving that the above system can comprehensively and rapidly evaluate the prognosis of chronic lymphocytic leukemia through multi-parameter flow cytometry analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com