Oligopeptide molecules, and preparation methods and applications thereof
An oligopeptide and molecular weight technology, applied in the protein field, can solve the problems of high recurrence rate and patient pain, and achieve the effect of obvious effect, obvious anti-inflammatory and analgesic, and anti-tumor immunity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of oligopeptide in embodiment 1 present invention
[0036] Raw material: Resin (Wang Resin), Fmoc protected amino acid,
[0037] Reagents: N,N-dimethylamide (DMF), DCM, MEOH, acetic anhydride, pyridine, DIEA, HBTU, hexahydropyridine
[0038] Instrument: 12-channel semi-automatic peptide synthesizer, high performance liquid chromatography (HPLC), model: Waters2695 Detection reagent: phenol reagent, pyridine, ninhydrin reagent
[0039] (1) The synthesis order is from C-terminal to N-terminal.
[0040] (2) Take 10mmol equivalent resin and put it into the polypeptide synthesizer reactor, add DCM (dichloromethane) to swell for half an hour, then remove the DCM, add 10mmol of the first Fmoc-protected amino acid in the sequence, 20mmol of DIEA (dichloromethane) Isopropylethylamine), an appropriate amount of DMF (dimethylformamide), DCM solution (appropriate amount means that the resin can be fully agitated), and react with nitrogen gas bubbling for 60 ...
Embodiment 2
[0048] Example 2 The effect of oligopeptide on the swelling degree of mouse auricle, the effect of anti-inflammation, detumescence and analgesia
[0049] Take Kunming mice, half male and half female, weighing 25-30 g, and randomly group them according to the sample size: 10 mice in each group. The doses of crude extracts of HY01-HY24 (250mg / kg) (2mg / ml), nabumetone (250mg / kg), and normal saline control group (0.1ml / 10g) were converted into the original animal crude drug doses (the same below). All groups were given oral administration, and the control group was given normal saline by oral administration. Each group was gavaged once a day for 4 consecutive days. 2 hours after the last gavage, Evan's blue 50mg / kg was injected into the tail vein, and 25ul of xylene was applied to both sides of the right ear of the mouse. Both ears were cut off from the baseline of the profile, and round ear pieces were punched on the same part of both ears with a puncher with a diameter of 7.5mm...
Embodiment 3
[0055] Embodiment 3 The influence of oligopeptide on the writhing response of mice caused by acetic acid, analgesic effect
[0056] Take Kunming mice, half male and half female, body weight 25-30g, randomly divided into groups, 14 oligopeptide administration group (250mg / kg), control group 5% aspirin soluble starch solution (250mg / kg), normal saline Group. 2 hours after the last gavage in the treatment group and the normal saline group, 0.6% glacial acetic acid was injected into the intraperitoneal cavity respectively, and the number of writhing caused by acetic acid in each group was observed within 15 minutes after giving 0.2ml of pain-inducing agent. The patients were treated as no writhing reaction.
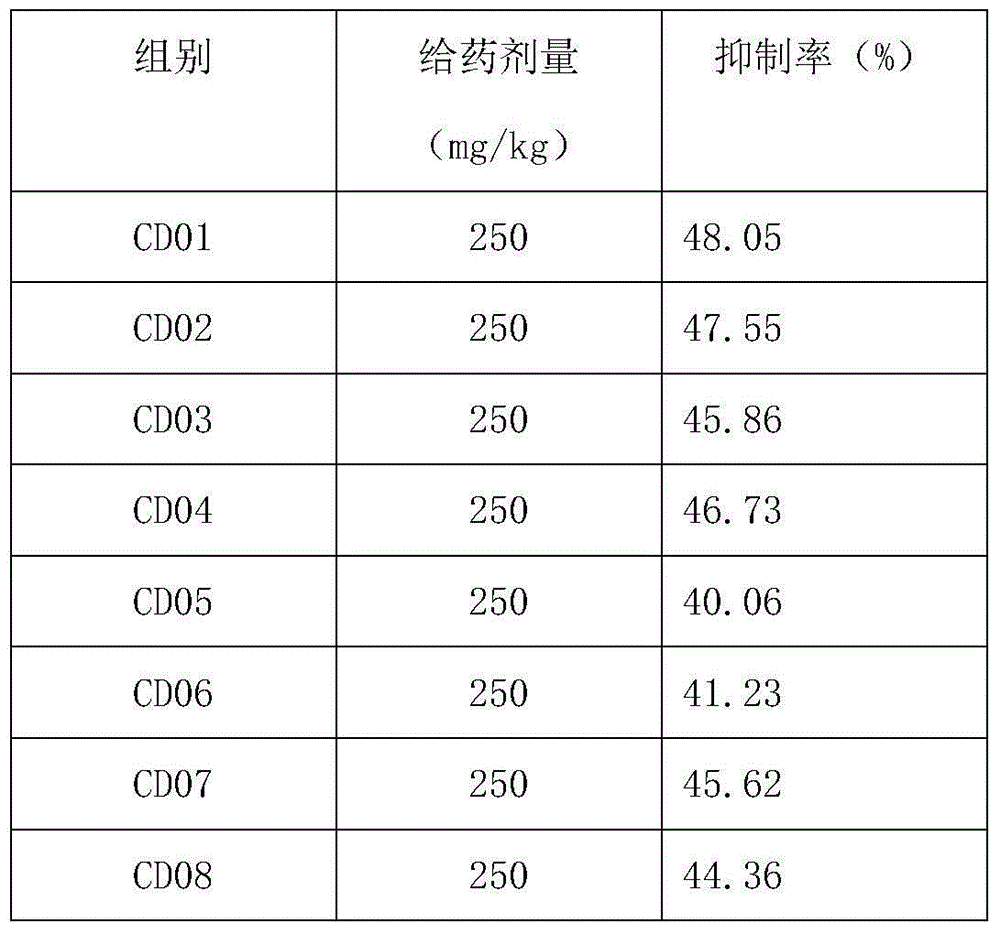
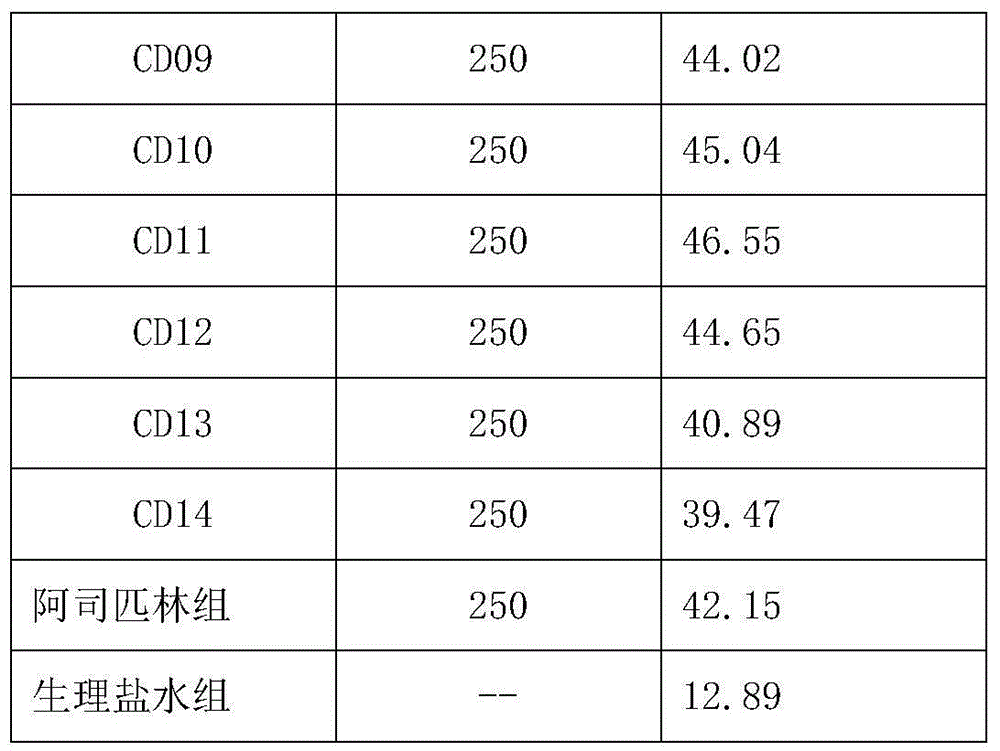
[0057] Table 2
[0058]
[0059]
[0060] The results showed that the oligopeptide group could effectively reduce the abdominal pain response to acetic acid in mice, among which CD01-CD04 and CD07-CD12 had better effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com