Anti-CD5 antibodies
An antibody, antibody molecule technology, applied in the direction of antibodies, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
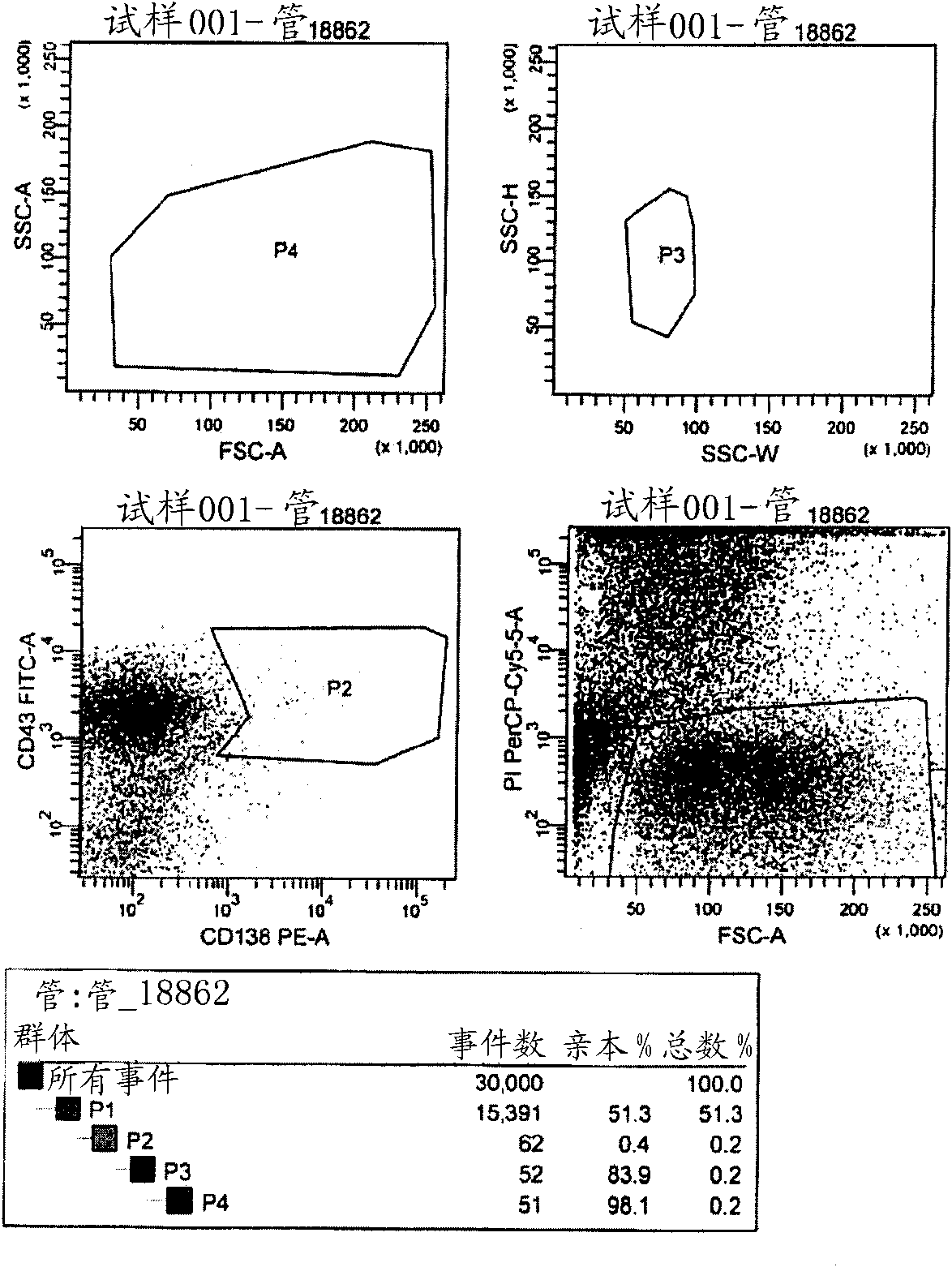
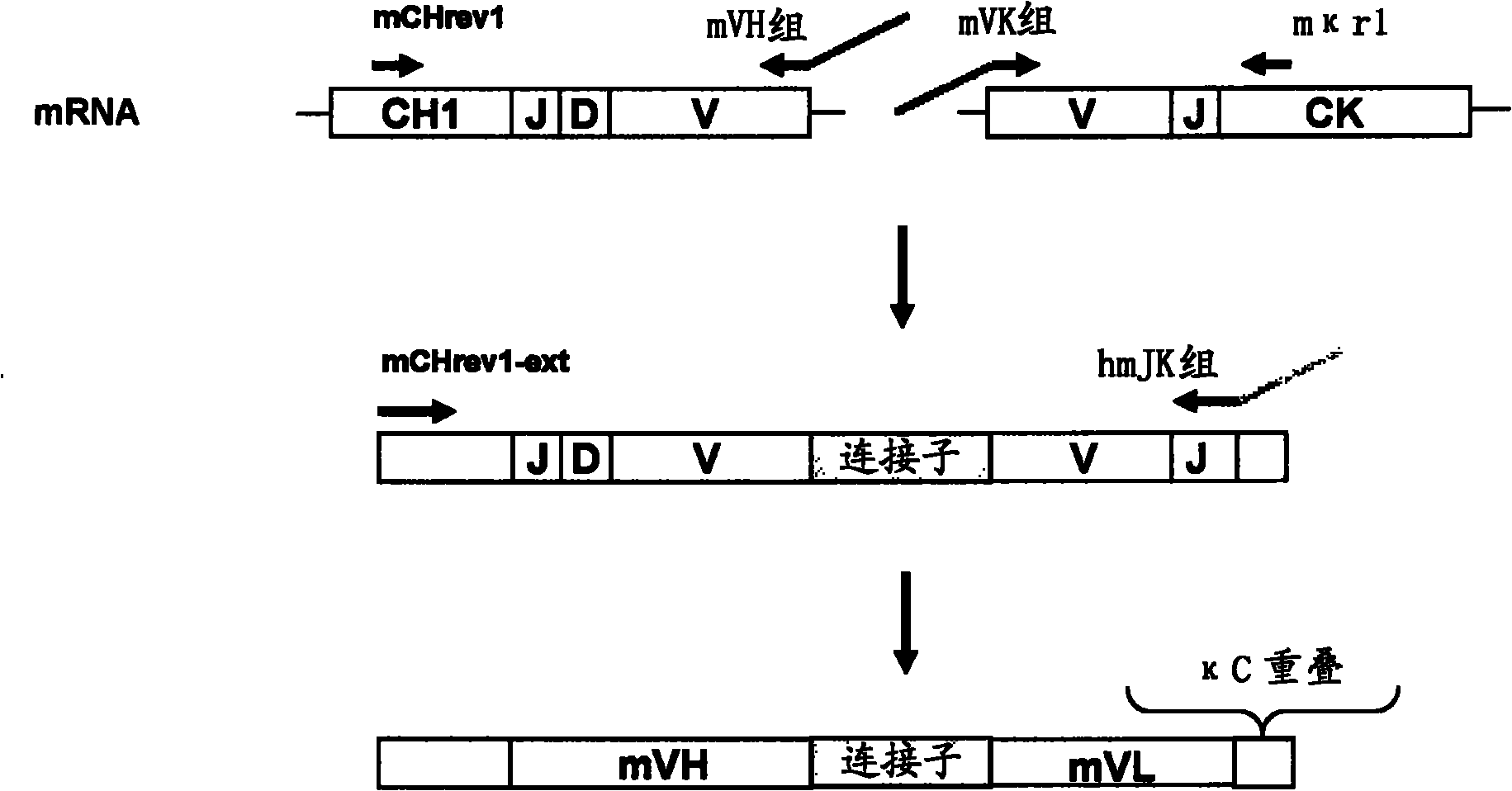
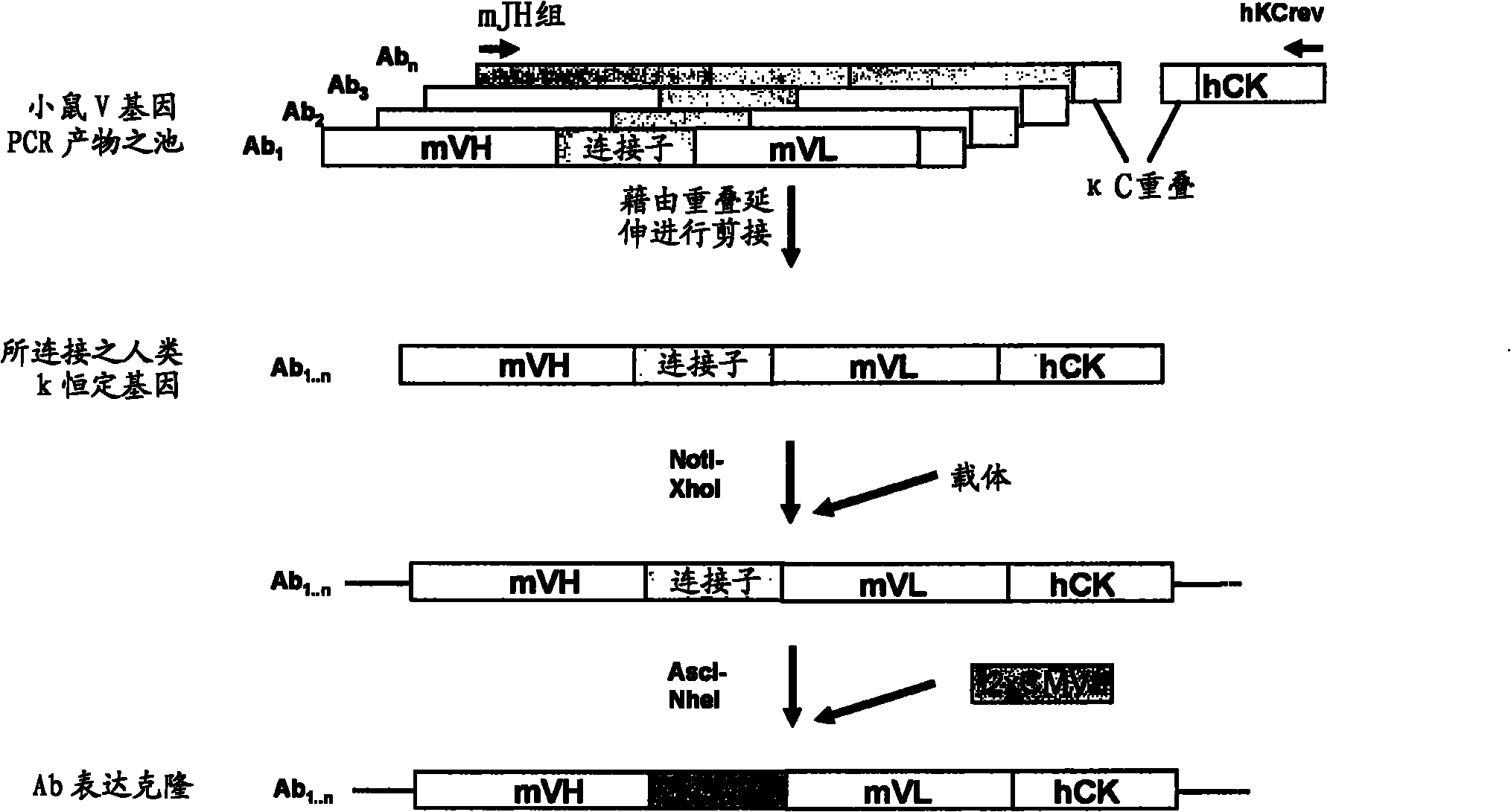
[0493] Example 1: Cloning of anti-CD5 antibodies
[0494] immunity
[0495] Female BALB / c strain A (8-10 weeks old) was used for immunization by injection of CD5-human growth hormone (hGH) fusion protein.
[0496] Recombinant CD5 extracellular domain (ECD) prepared in-house was used for all immunizations. CD5-ECD was produced as a fusion protein consisting of the ECD of CD5 and human growth hormone (hGH), isolated by the tobacco etch virus (TEV) cleavage site.
[0497] CD5-hGH was diluted in PBS and then mixed 1:1 with Freund's Adjuvant. Adjuvants are used to enhance and modulate the immune response. For the first immunization, complete FA's adjuvant (CFA) was used, while incomplete FA's adjuvant (IFA) was used for subsequent immunizations. IFA is an oil-in-water emulsion containing mineral oil and CFA is IFA with added heat to kill dry mycobacterial species. Both adjuvants have a reservoir effect. CFA produces a long-lasting immune response and is used in the first ...
Embodiment 2
[0531] Example 2: Mammals prepare anti-CD5 antibodies
[0532] Anti-EGFR antibodies were transiently expressed using the Freestyle MAX CHO Expression System (Invitrogen). Antibodies were expressed in volumes of 200-2000ml.
[0533] About 24 hours before transfection, subculture CHO-S cells to reach 0.5×10 6 cell concentration per cell / ml. Plasmid was diluted in OptiPro serum-free medium (1.25 μg per ml of cell culture medium) and mixed with a solution of FreeStyle MAX transfection reagent as recommended by the supplier. The transfection reagent was transferred to the cell culture and the supernatant was collected 8 days later.
[0534] The expressed antibodies were purified from the culture supernatants using an affinity chromatography step using a protein A sepharose column (MabSelect Sure, GE Health Care) to purify IgGl molecules. Antibody was eluted from the column using 0.1 M Glycine 2.7. Antibody-containing fractions, as determined by absorbance measurements at 280...
Embodiment 3
[0535] Example 3: Determining Epitope Specificity
[0536] Competition ELISA using reference antibody
[0537] A competition ELISA that distinguishes the binding epitopes of anti-CD5 antibodies was developed by using a reference antibody that binds CD5 by incubation with a secondary reagent that is specific for the human Fc region of an anti-CD5 antibody and does not display Cross activity with murine IgG Fc. The ELISA was adapted from the description published in Ditzel et al., 1995, The Journal of Immunology, Vol. 154, No. 2, 893-906.
[0538] Epitope blocking ELISA was performed by diluting CD5ECD antigen to 1 μg / ml in PBS and coating 50 μl / ELISA well overnight at 4°C. The next morning, wells were washed twice with PBS-T and blocked with PBS-T-1% BSA for 1 hour at room temperature, followed by 4 washes in PBS-T. Next, 25 μl of murine reference mAbs were added to individual ELISA wells at dilutions known from previous experiments to saturate all epitopes on CD5 at this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com