Compositions and methods for the depletion of cd5+ cells
a technology of cd5+ cells and cd5+ cells, which is applied in the field of compositions and methods for the depletion of cd5+ cells, can solve the problems of serious complications, impede the use of hematopoietic engraftment in the clinic, and difficulty in ensuring engraftment of hematopoietic cells, so as to prevent or reduce the likelihood of rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
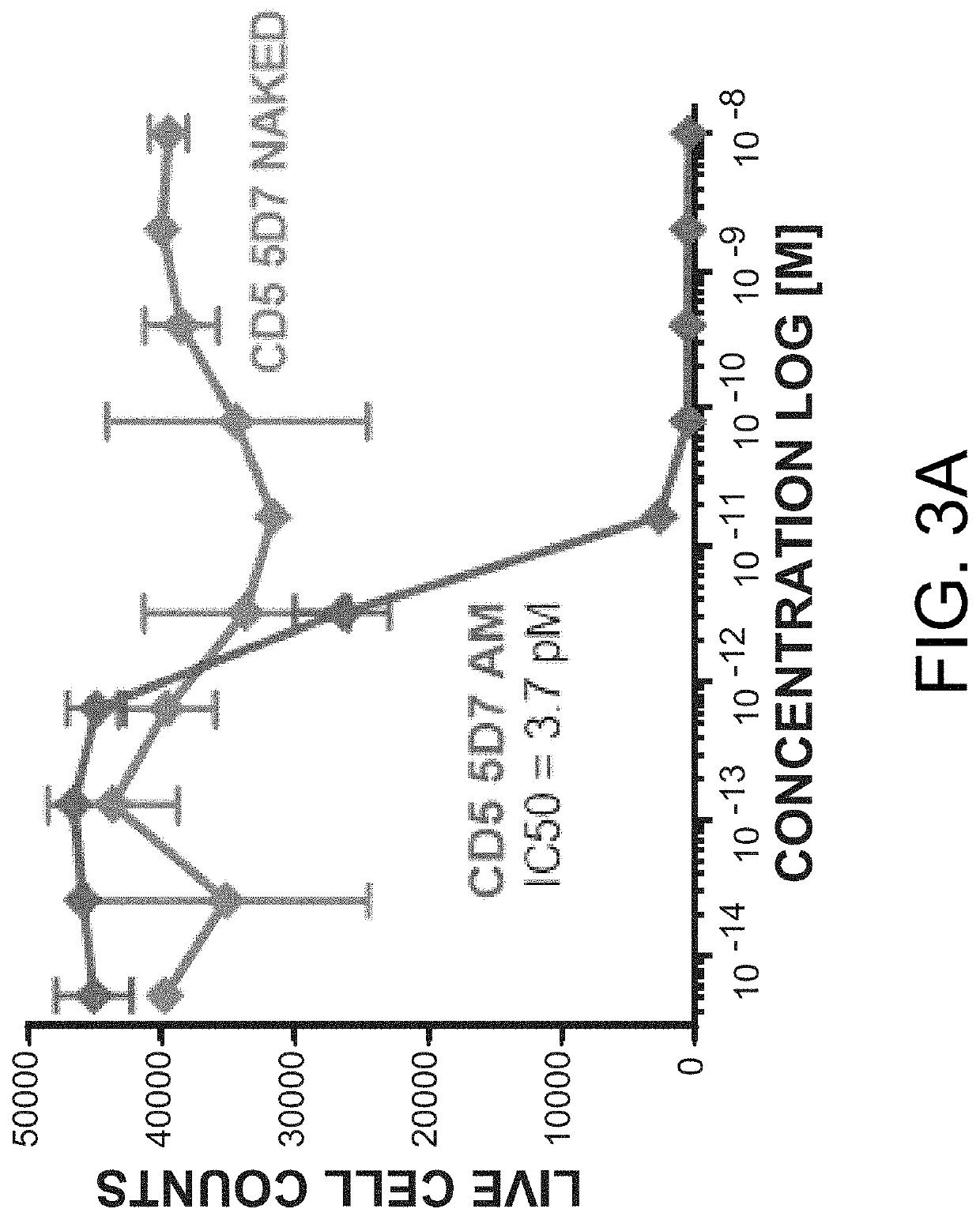
Examples
example 1
Binding Analysis of Anti-CD5 Antibodies
[0701]To determine the binding characteristics of anti-CD5 antibody 5D7 hIgG1, antibody binding studies were performed at 25 degrees Celsius in 1×PBS supplemented with 0.1% w / v bovine serum albumin with a Pall ForteBio Octet Red96 using biolayer interferometry (BLI). The purified human anti-CD5 antibody (5D7) was immobilized onto anti-human Fc biosensors (AHC; Pall ForteBio 18-5063) and incubated with 50 nM of purified human CD5 ectodomain). The binding characteristics of anti-CD5 antibody 5D7 are shown in Table 3. Anti-human CD5 antibody 5D7 as used in Examples 1 to 5 is a humanized version of murine antibody 5D7 (see US 2008 / 0254027). The sequences of antibody 5D7 as used herein are described in SEQ ID Nos: 257 and 258 (heavy and light chain variable region amino acid sequences) and SEQ ID Nos: 29 to 34 (heavy and light chain CDRs).
TABLE 3Binding kinetics of 5D7 to human CD5 ectodomainConc.ResponseKD KONKDIS Full Antibody(nM)(nm)(M)(1 / Ms)(1 / s...
example 2
Cell Line Binding Analysis of Anti-CD5 Antibodies
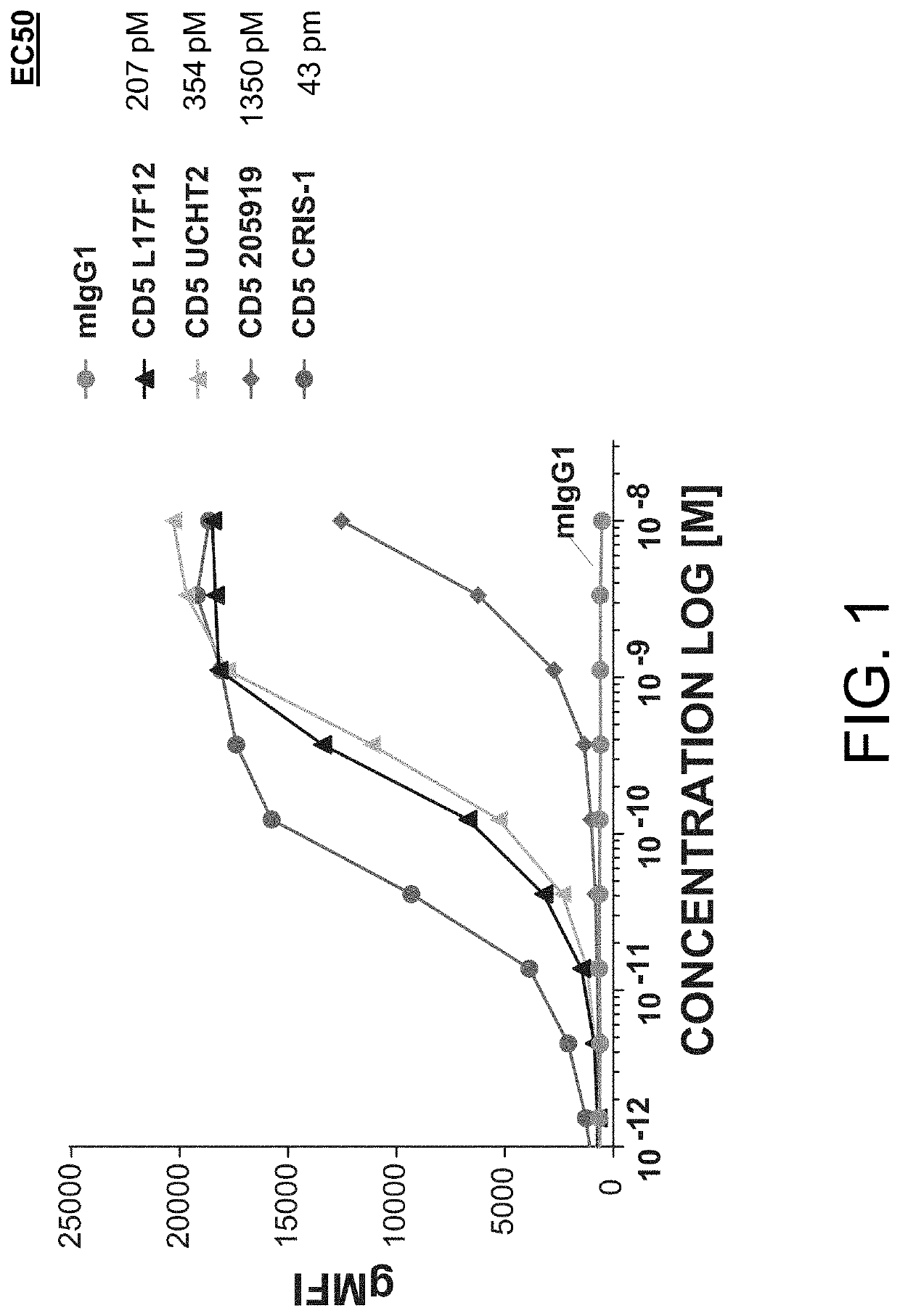
[0702]MOLT-4 cells (i.e., an immortalized human T lymphoblast cell line) were plated at 20,000 cells / well and stained with a titration of the indicated murine anti-CD5 antibodies (i.e., L17F12, UCHT2, 205919, and CRIS-1) for 2 hours at 4° C. Secondary anti-mouse AF488 stain, at a constant amount, was added for 30 minutes at 4° C. After washing, plates were run on a flow cytometer and binding of the indicated antibody (and the negative control, i.e., mIgG1) was determined based on geometric mean fluorescence intensity in the AF488 channel. Results from these assays are provided in FIG. 1.
[0703]As shown in FIG. 1, the murine anti-CD5 antibodies L17F12 (Thermo Fisher), UCHT2 (BioLegend), 205919 (Novus Biologicals), and CRIS-1 (Novus Biologicals) bound to human T lymphoblast cells (i.e. MOLT-4 cells), with an EC50=207 pM (L17), 354 pM (UCH), 1350 pM (205), and 43 pM (CRIS).
example 3
Primary Cell Binding Analysis of Anti-CD5 Antibodies
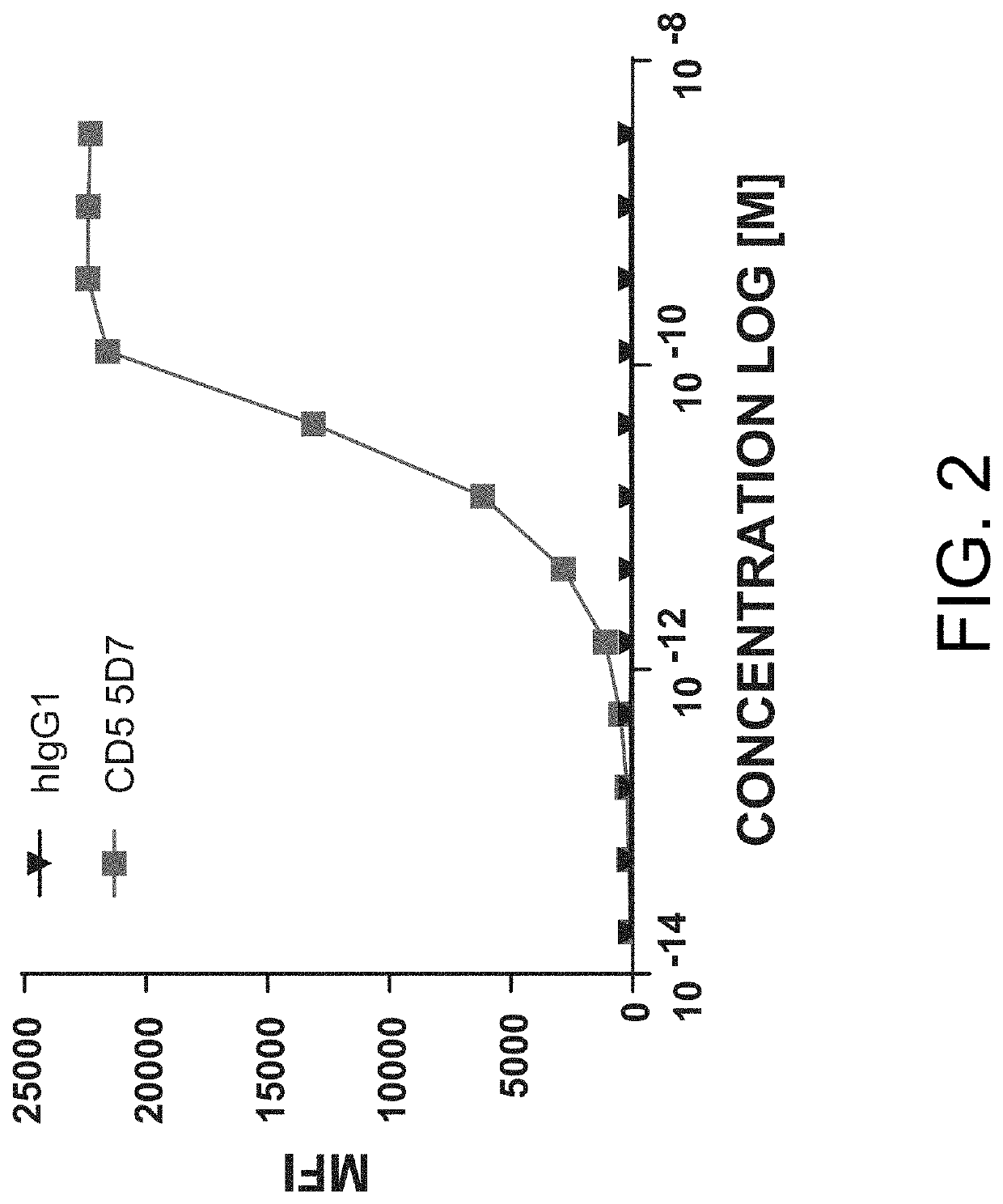
[0704]Primary human T-cells were plated at 8×104 cells / well and stained with a titration of the human anti-CD5 antibody 5D7 for 2 hours at 37° C. Secondary anti-mouse AF488 stain, at a constant amount, was added for 30 minutes at 4° C. After washing, plates were run on a flow cytometer and binding of the anti-CD5 5D7 antibody (and the negative control, i.e., hIgG1) was determined based on geometric mean fluorescence intensity in the AF488 channel. Results from these assays are provided in FIG. 2.
[0705]As shown in FIG. 2, the anti-CD5 antibody 5D7 bound to primary human T-cells with an EC50=3.0 pM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com