T cell depletion therapy
A cell and cytotoxin technology, applied in chemical instruments and methods, medical raw materials derived from mammals, allergic diseases, etc., can solve problems such as patient illness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0738] Preparation of antibody-drug conjugates
[0739] Among the ADCs, the antibody or antigen binding fragment thereof is conjugated to one or more cytotoxic drug moieties (D), such as one or more cytotoxic drug moieties (D), as disclosed herein. 20 drug parts. The ADCs of the present disclosure can be prepared by a variety of pathways, and an organic chemical reaction, conditions, and reagents known to those skilled in the art, including: (1) antibody or antigen-binding fragment reactive substituent reaction formation with a divalent ligation reagent As described above, the AB-ZL described above is then reacted with the drug portion D; or the reactive substitution of the drug portion reacts with the divalent joint reagent to form a DLZ, then the antibody as described above or its antigen binding fragment. The reactive substituent reaction has formed an ADC of formula DLZ-AB, such as AM-ZL-AB. Further methods of preparing ADCs are described herein.
[0740] In another aspect, th...
Embodiment 1
[0777] Example 1: In vitro binding analysis of anti-CD2 antibody.
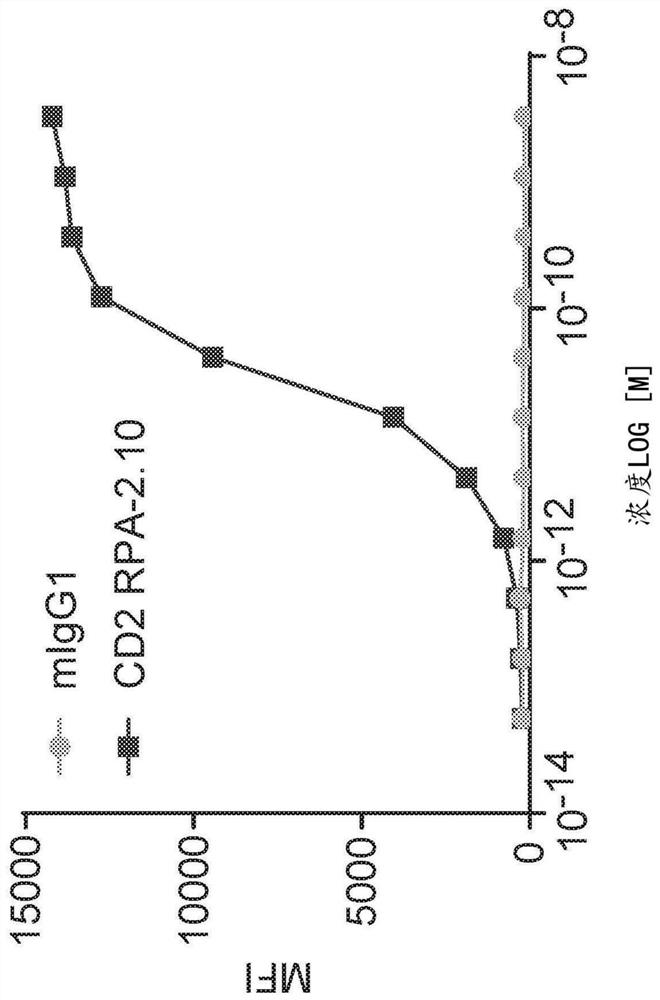
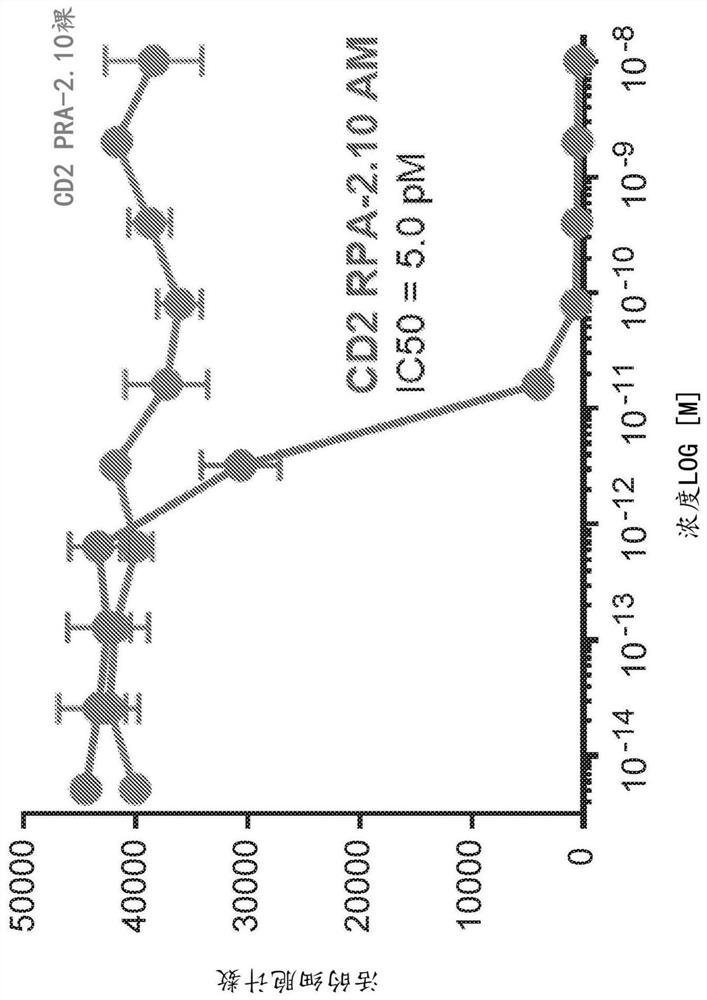
[0778] In order to determine the binding features of anti-CD2 antibody RPA-2.10 migg1 and AB1 HIGG1, at 25 degrees Celsius (BLI) using the PALLTEBIO OCTET RED96 using the PALLTEBIO OCTET RED96 in 1 xpbs added 0.1% W / V borey albumin. Antibody binding studies. The designated purified human (AB1-HIGG1) antibody was immobilized in an anti-human FC biosensor (AHC; PALLTEBIO 18-5063) or a mouse Fc biosensor (AMQ; PallFortebio 18-5090) Extracellular and EtOAc EtOAc EtOAc EtOAc EtOAc. Apparent single price affinity (K D ), Apparent binding rate (k ON ) And apparent dissociation rate (K DIS The partial fully fitted to the 1: 1 binding model is determined, such as the calculation of each IgG and purified human CD2 extraordinary domain, as shown in Table 3, is shown in Table 3.
[0779] Further characterization of anti-CD2 antibodies is provided in Examples 2 to 6.
[0780] table 3: Combined kinetics of designated IgG and...
Embodiment 2
[0782] Example 2: In vitro cell line combination analysis of anti-CD2 antibody
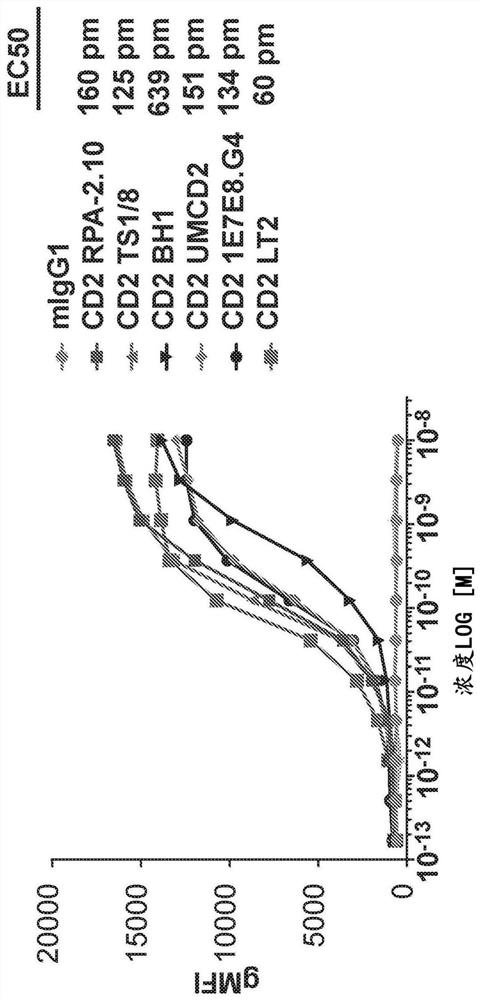
[0783] Molt-4 cells (i.e.. G4 or LT2) was stained at 4 ° C for 2 hours. A constant amount of secondary anti-mouse AF488 stain was added at 4 ° C for 30 minutes. After washing, the board operates on a streaming cytometry and determines the binding of the specified antibody (and negative control, MIGG1) based on geometric average fluorescence intensity in the AF488 channel. figure 1 The results of these assays are provided.
[0784] Such as figure 1 As shown, the mouse anti-CD2 antibody RPA-2.10, TS1 / 8, BH1, UMCD2, 1E7E8.G4 and LT2 combined with human T lymphobocytes (ie Molt-4 cells), EC 50 = 160 pm (RPA-2.10), 125PM (TS1 / 8), 639PM (BH1), 151PM (UMCD2), 134PM (1E7E8) and 60PM (LT2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com