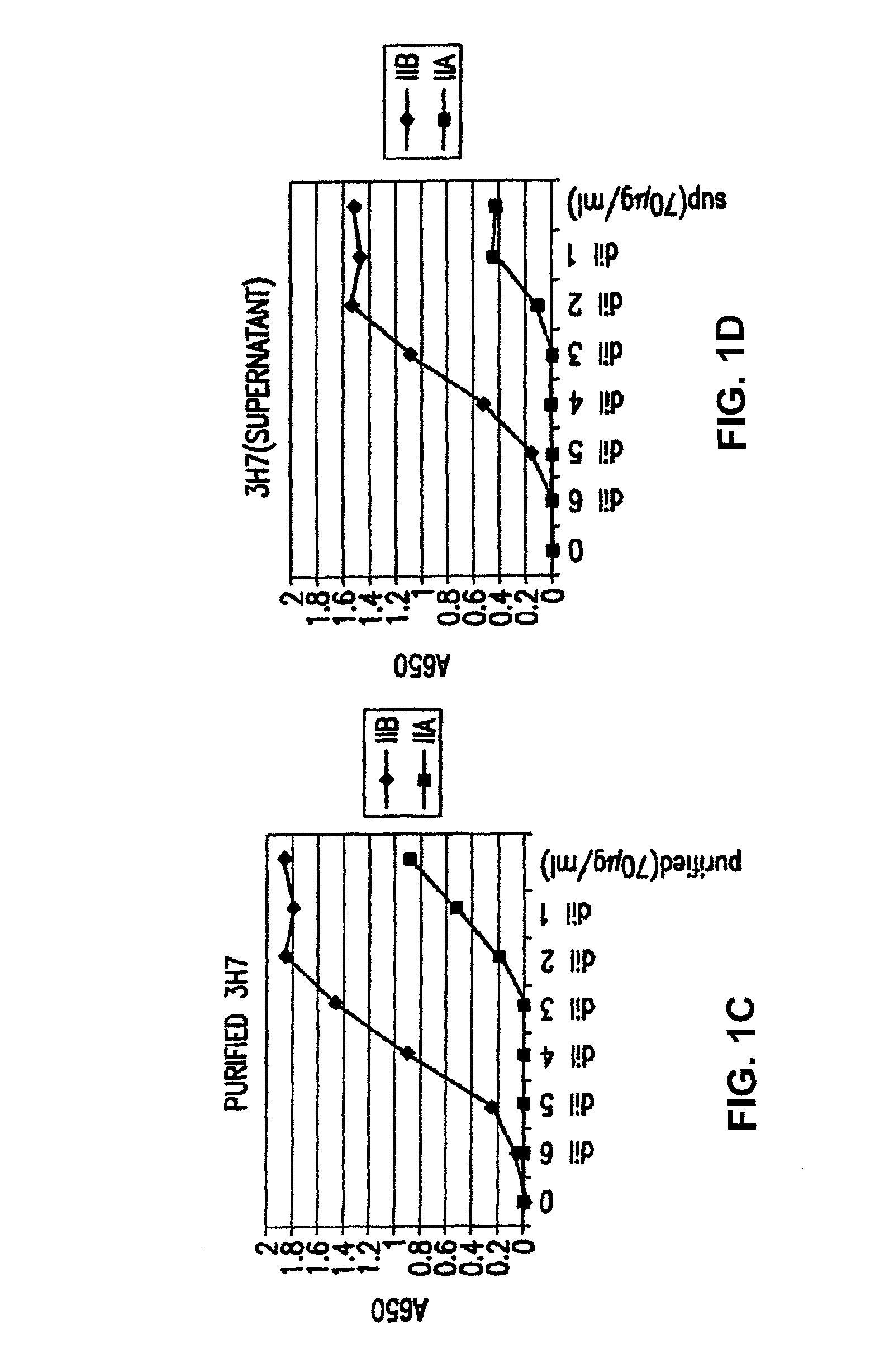
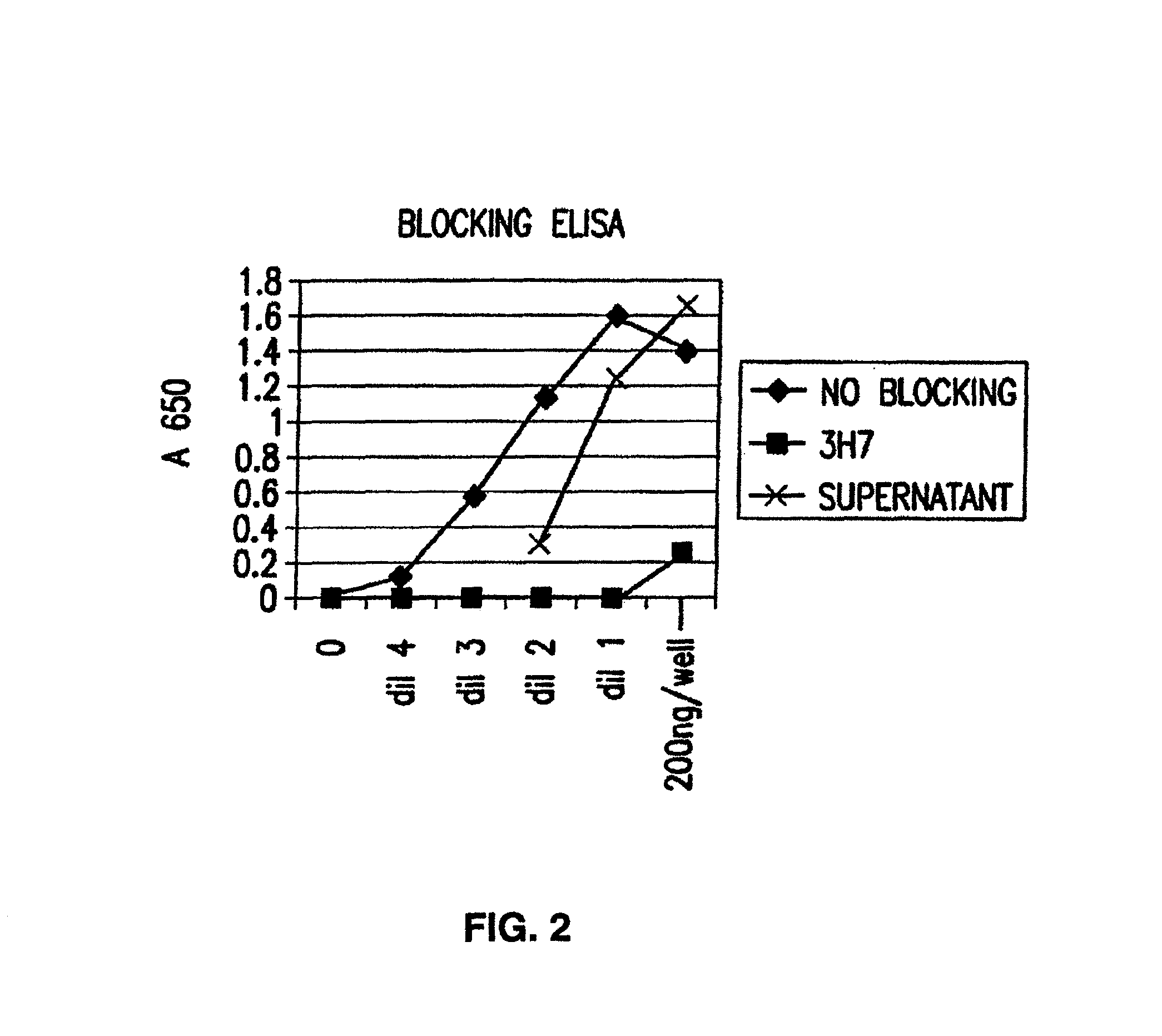
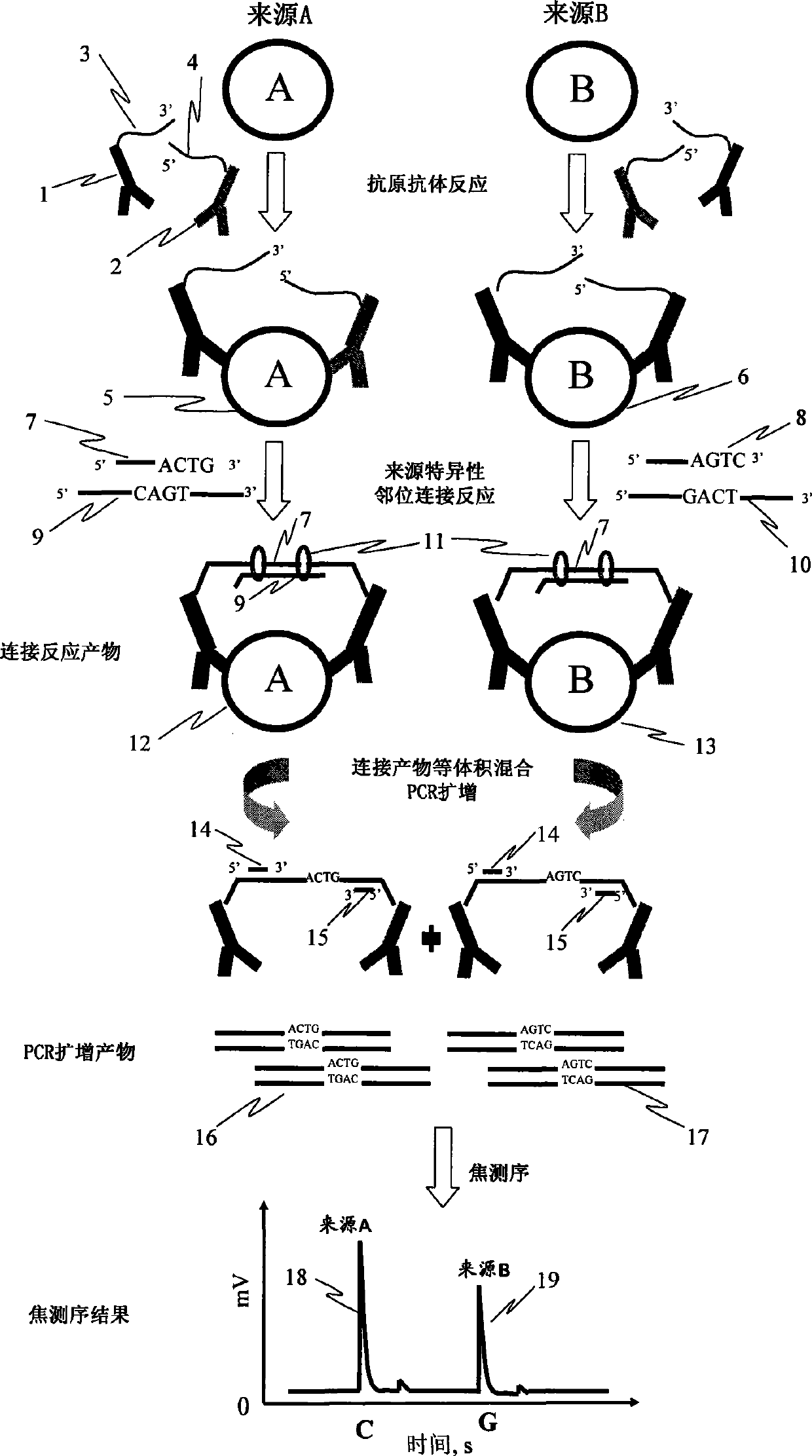
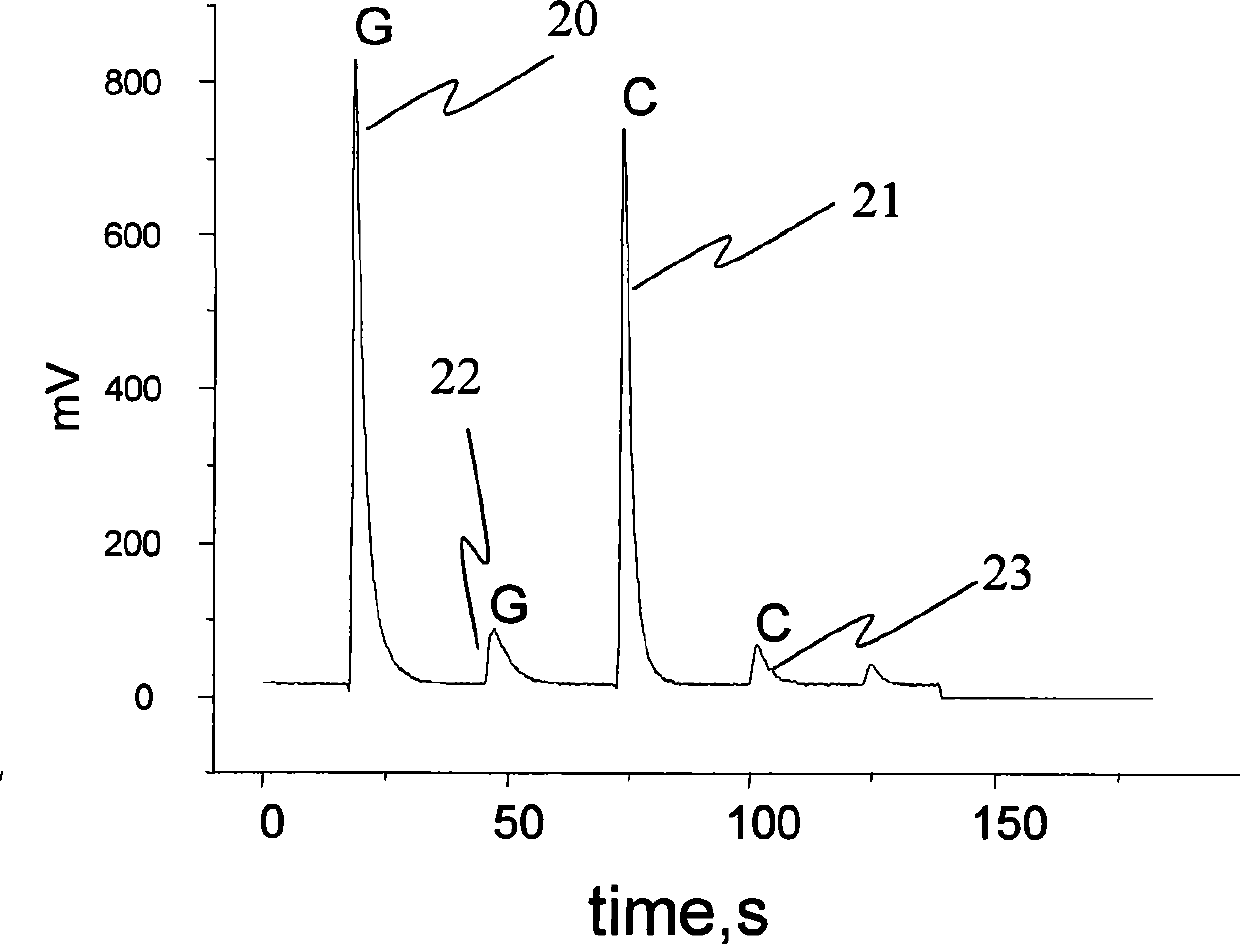
Patents
Literature
120 results about "Antigen-Antibody Complex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The complex formed by the binding of antigen and antibody molecules. The deposition of large antigen-antibody complexes leading to tissue damage causes IMMUNE COMPLEX DISEASES.
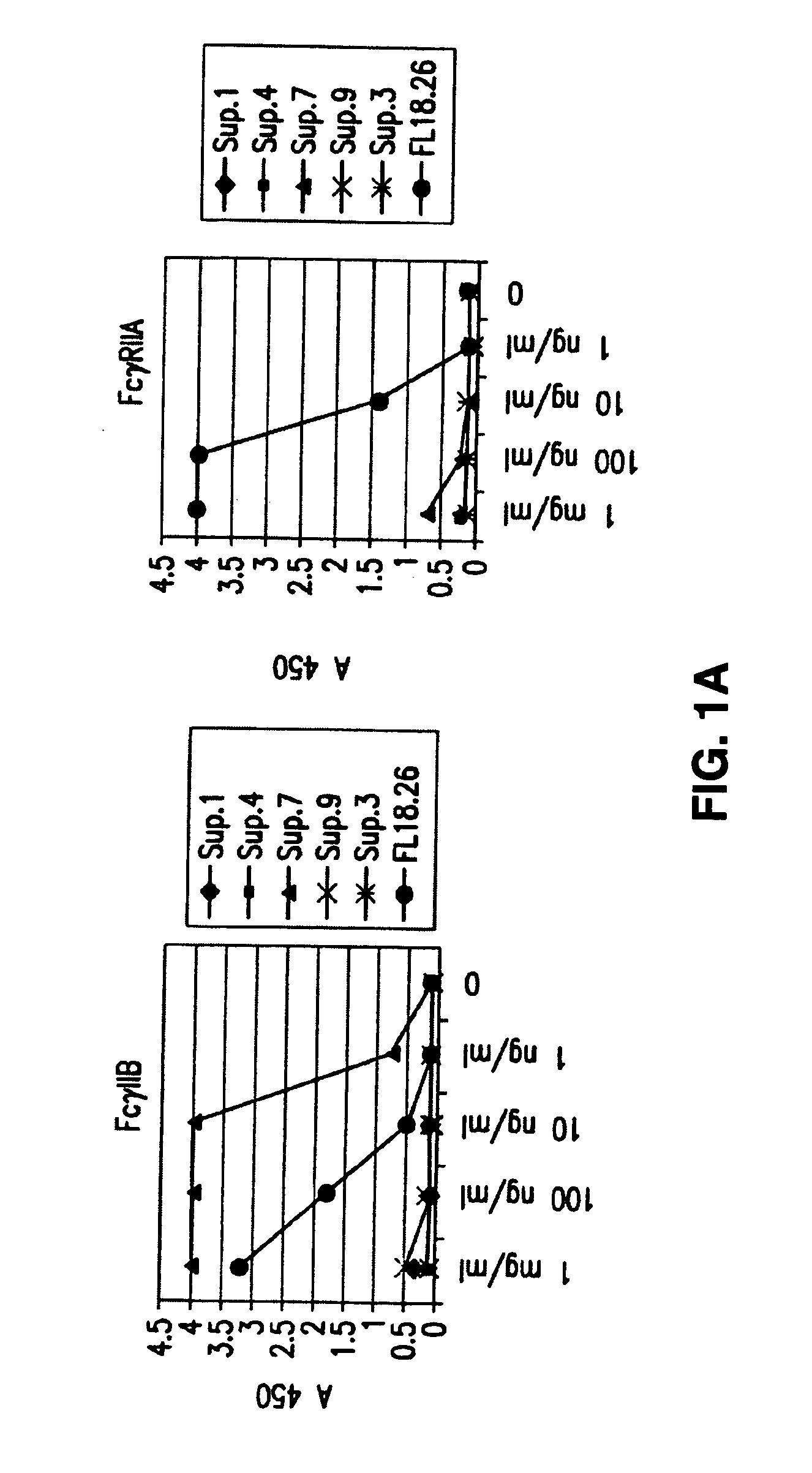
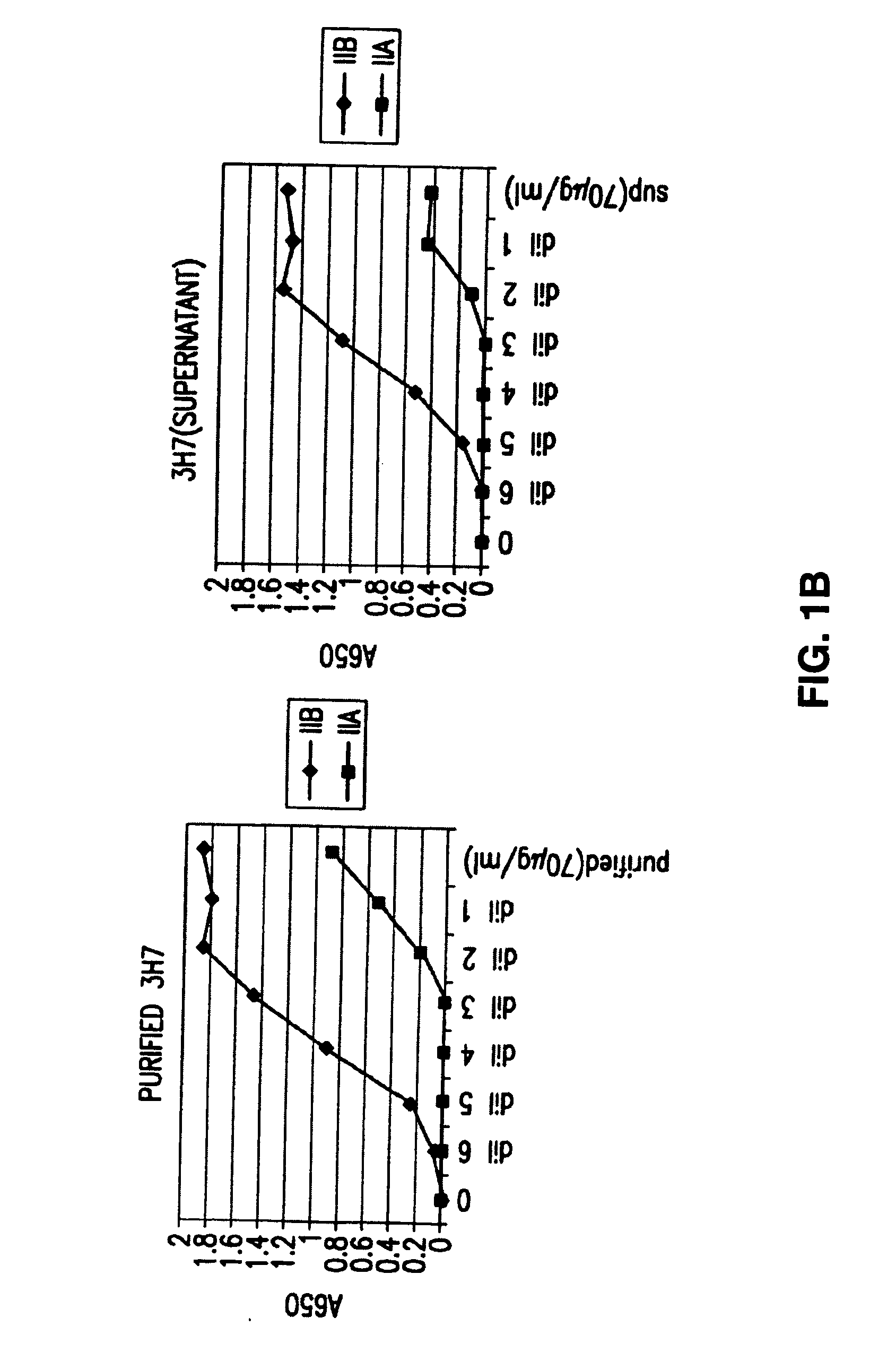
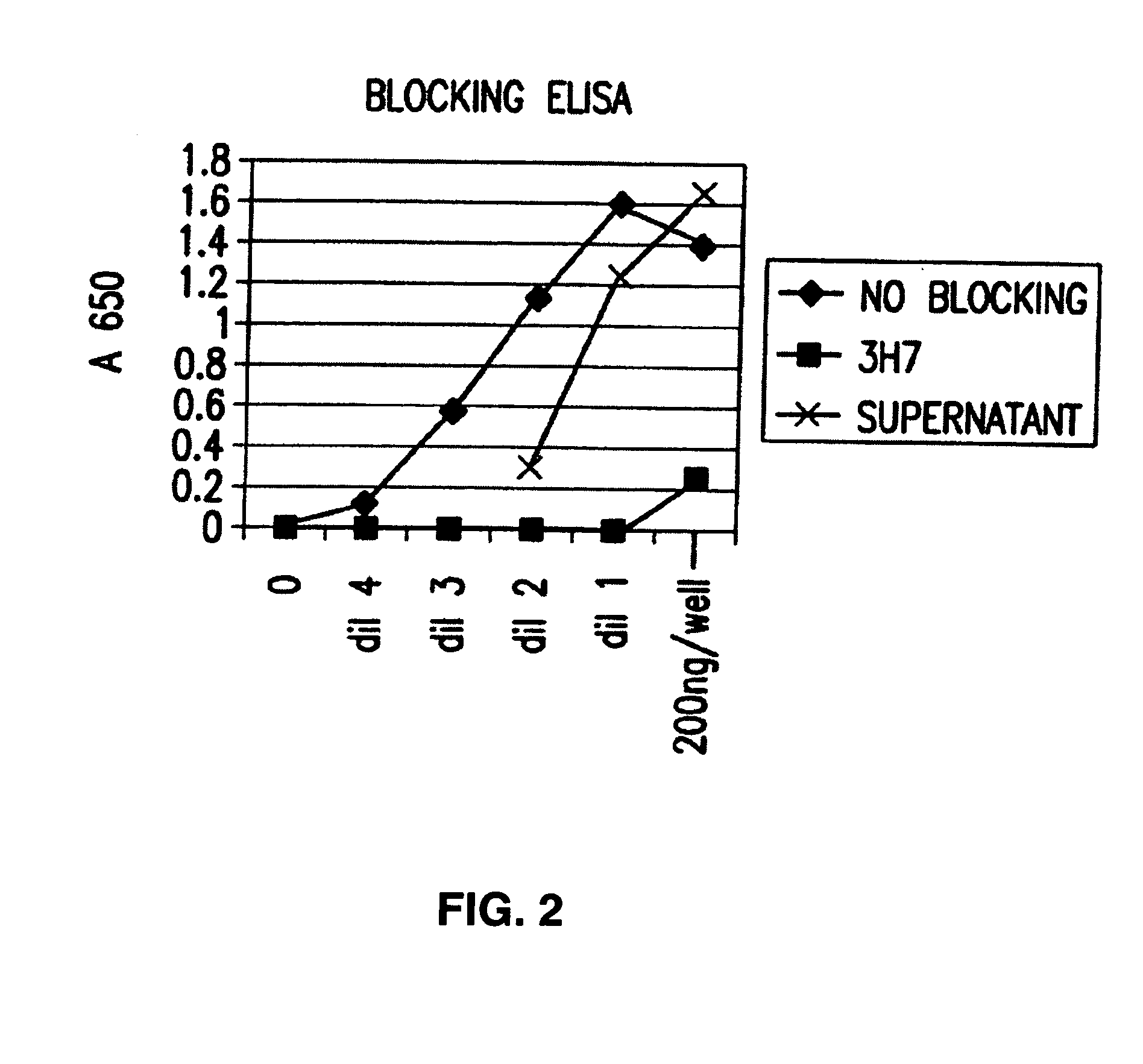
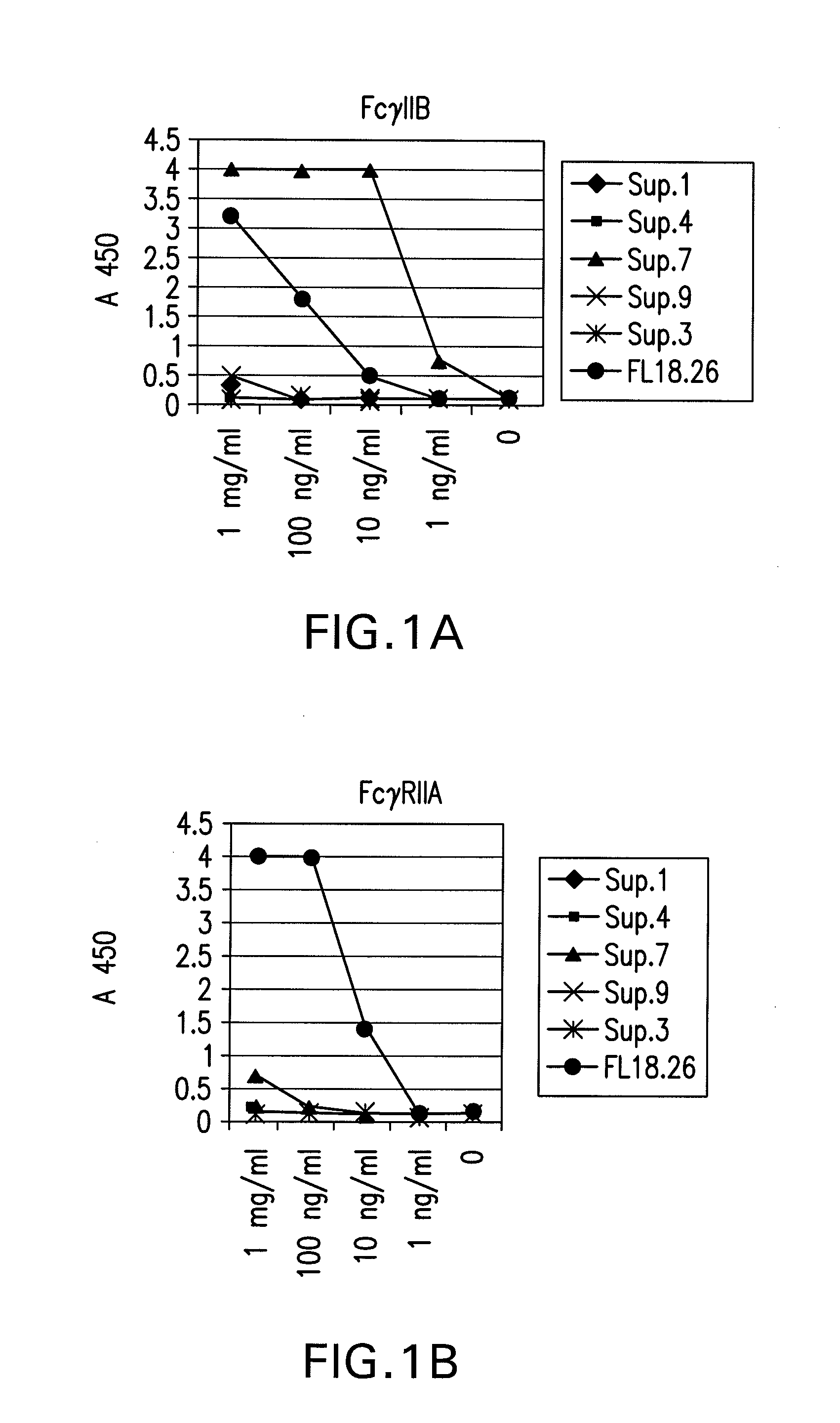
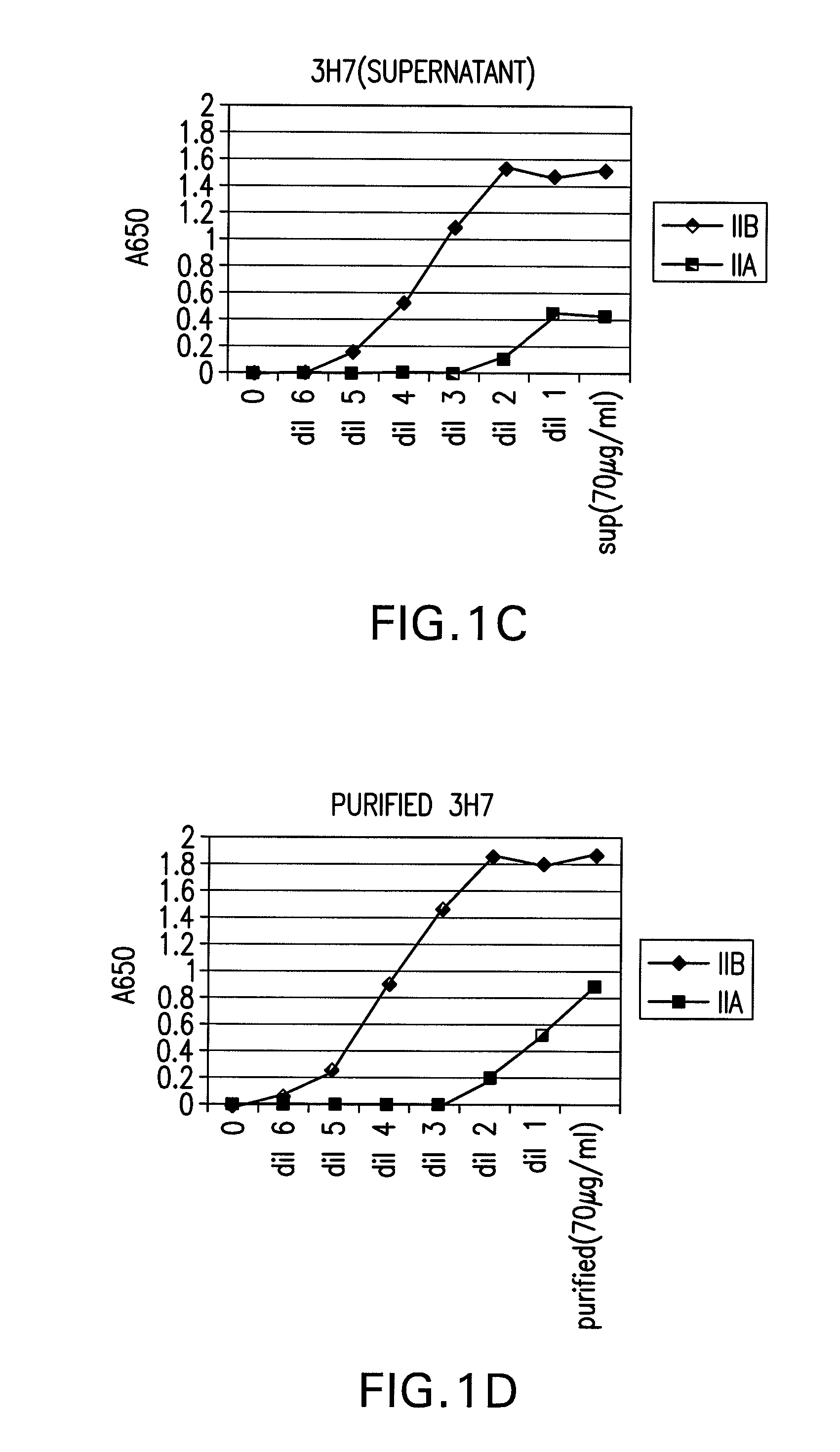
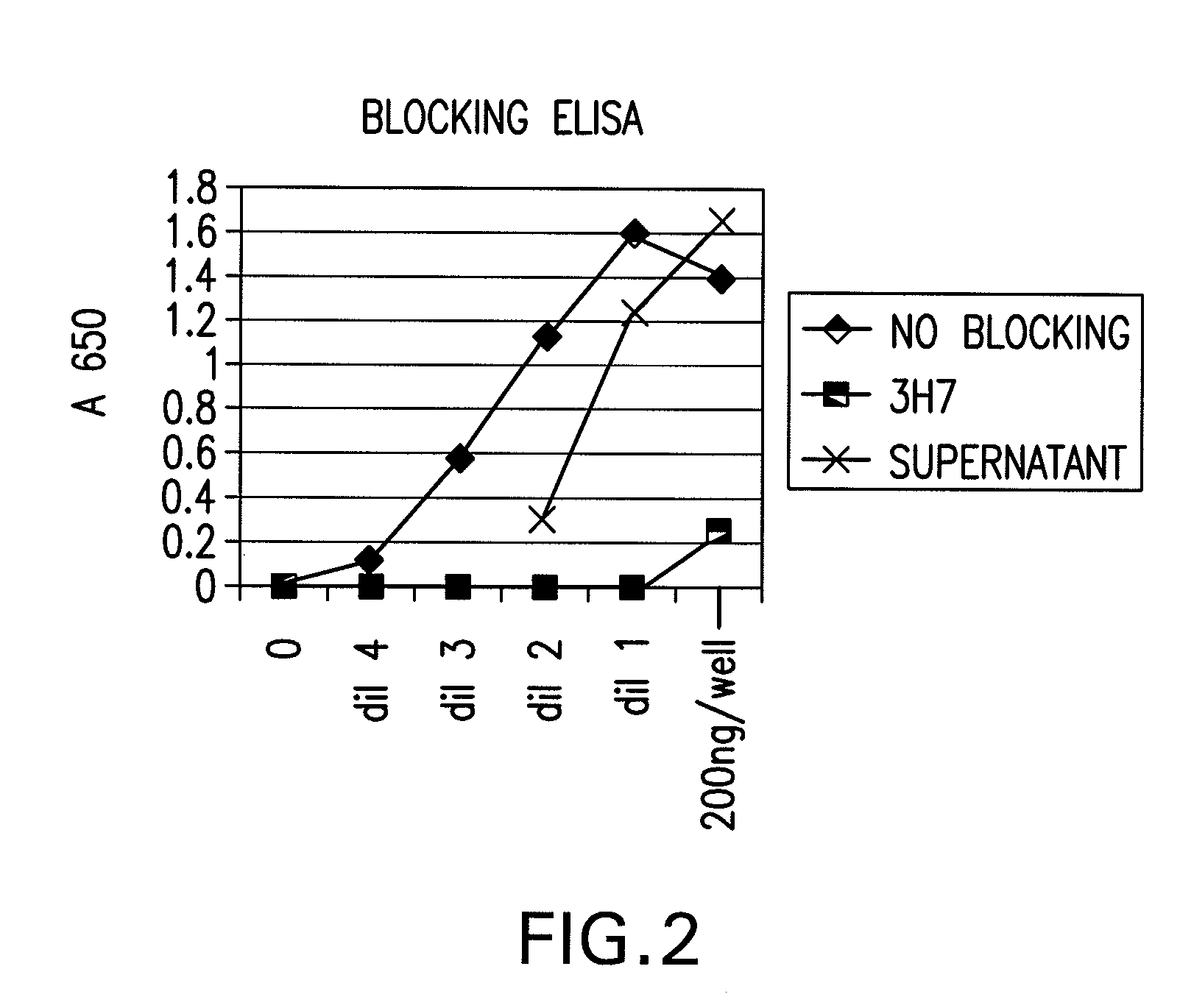
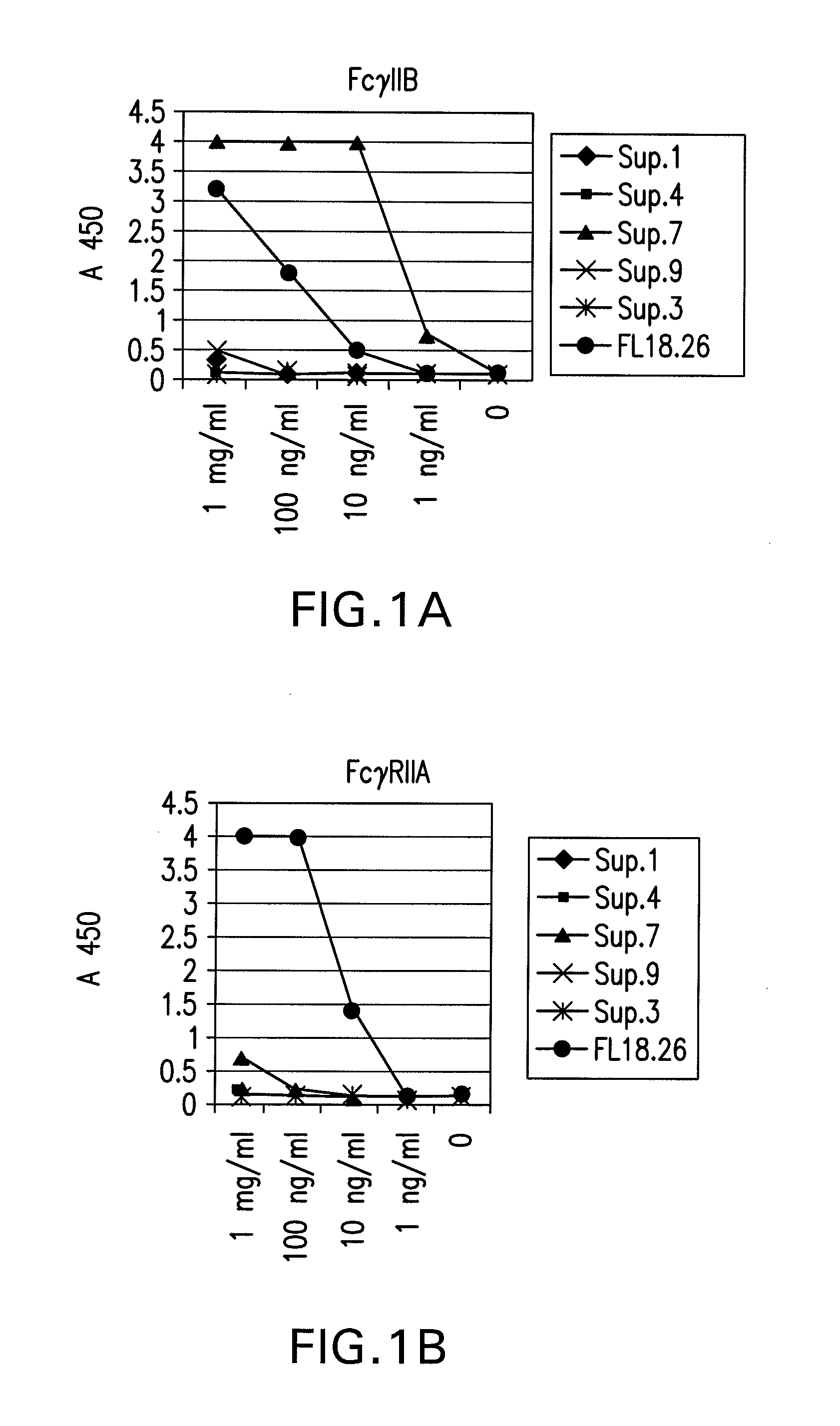
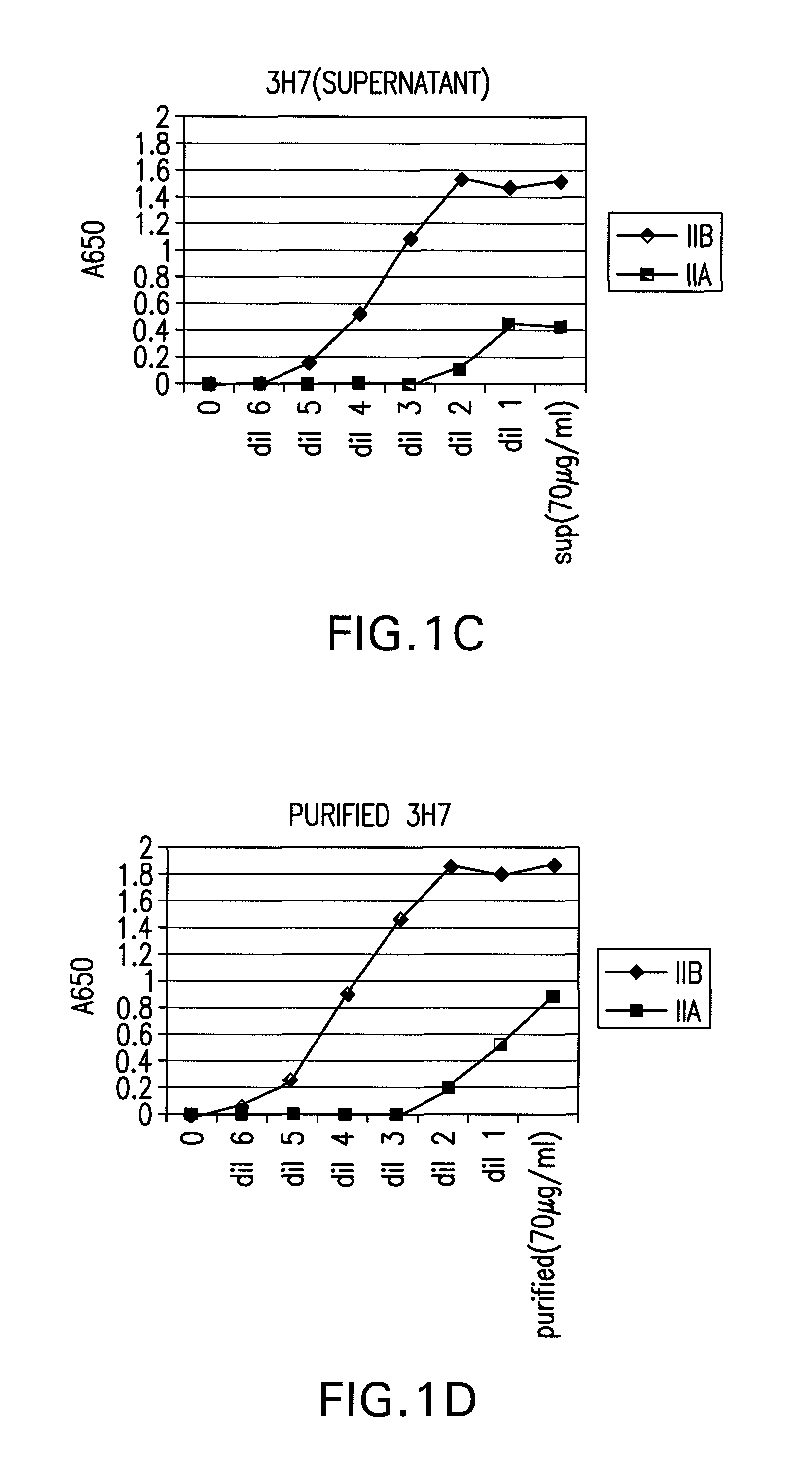
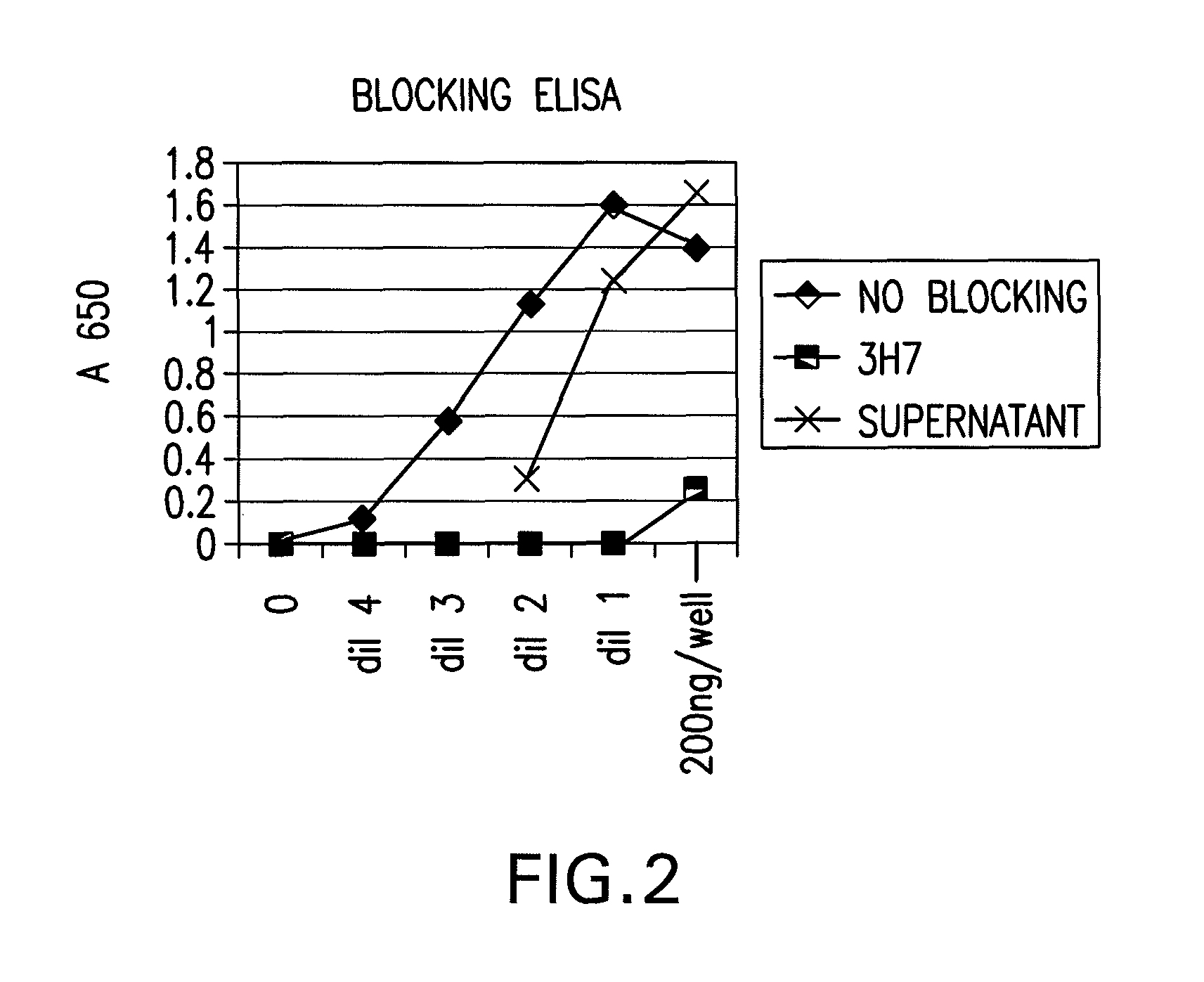
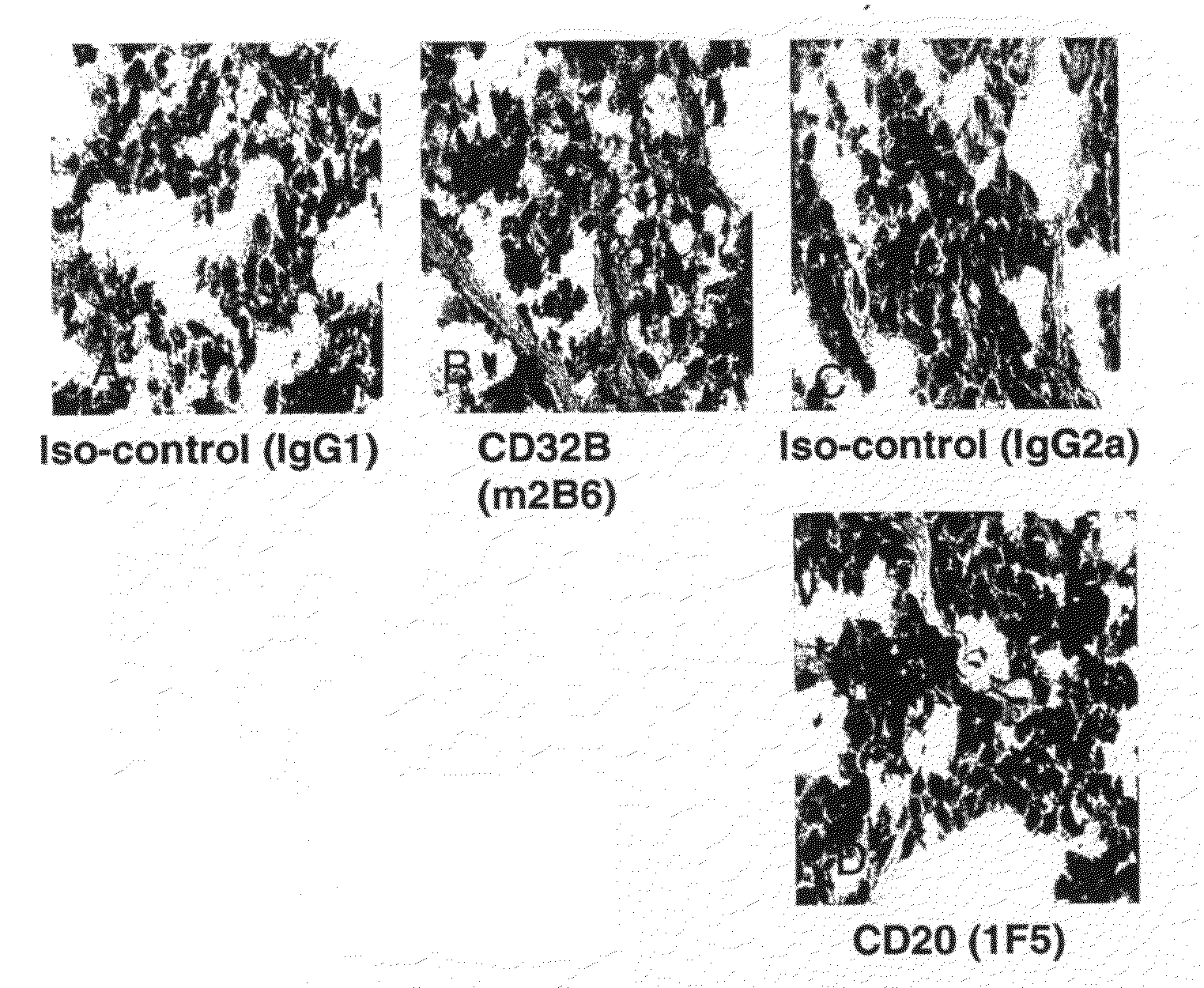
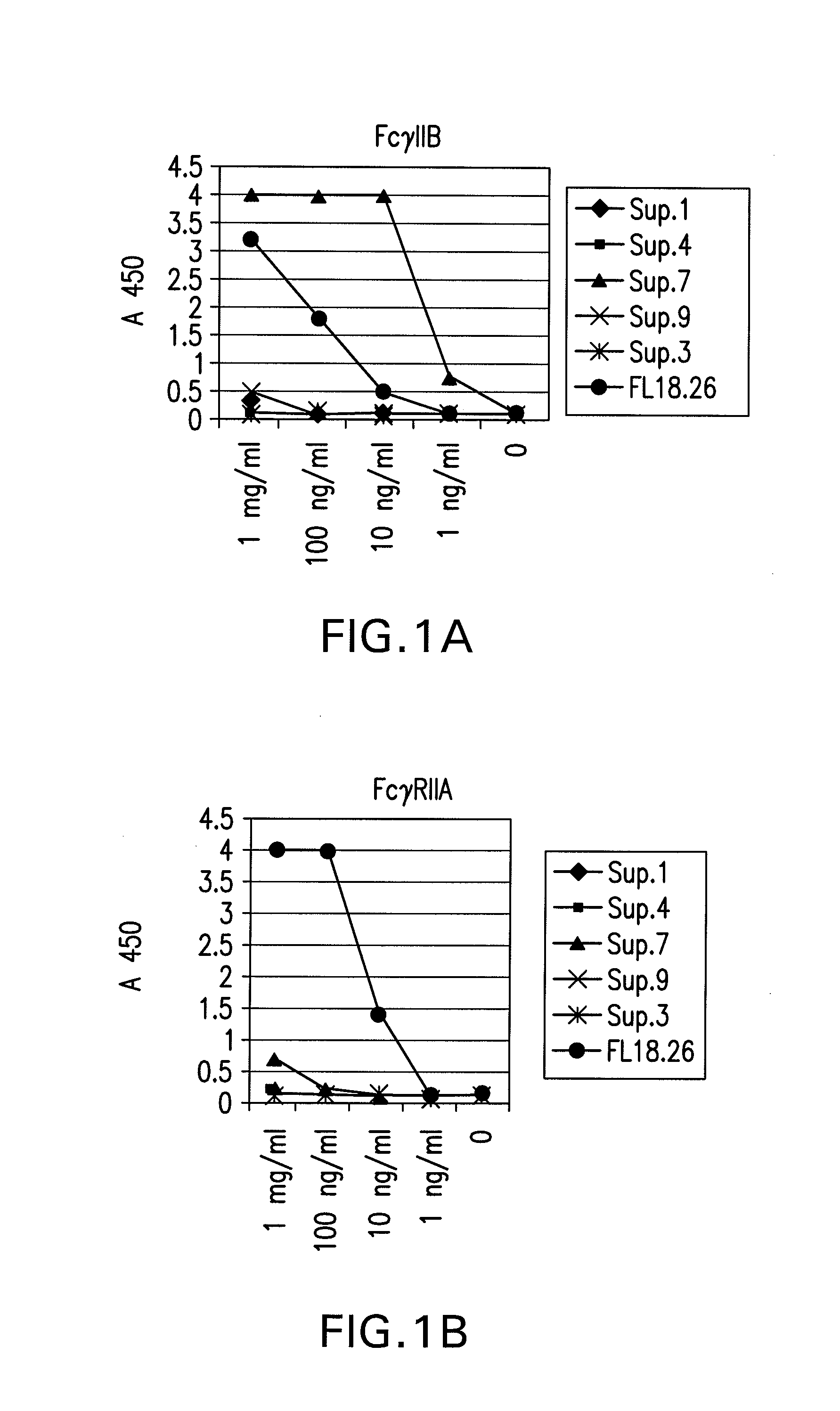
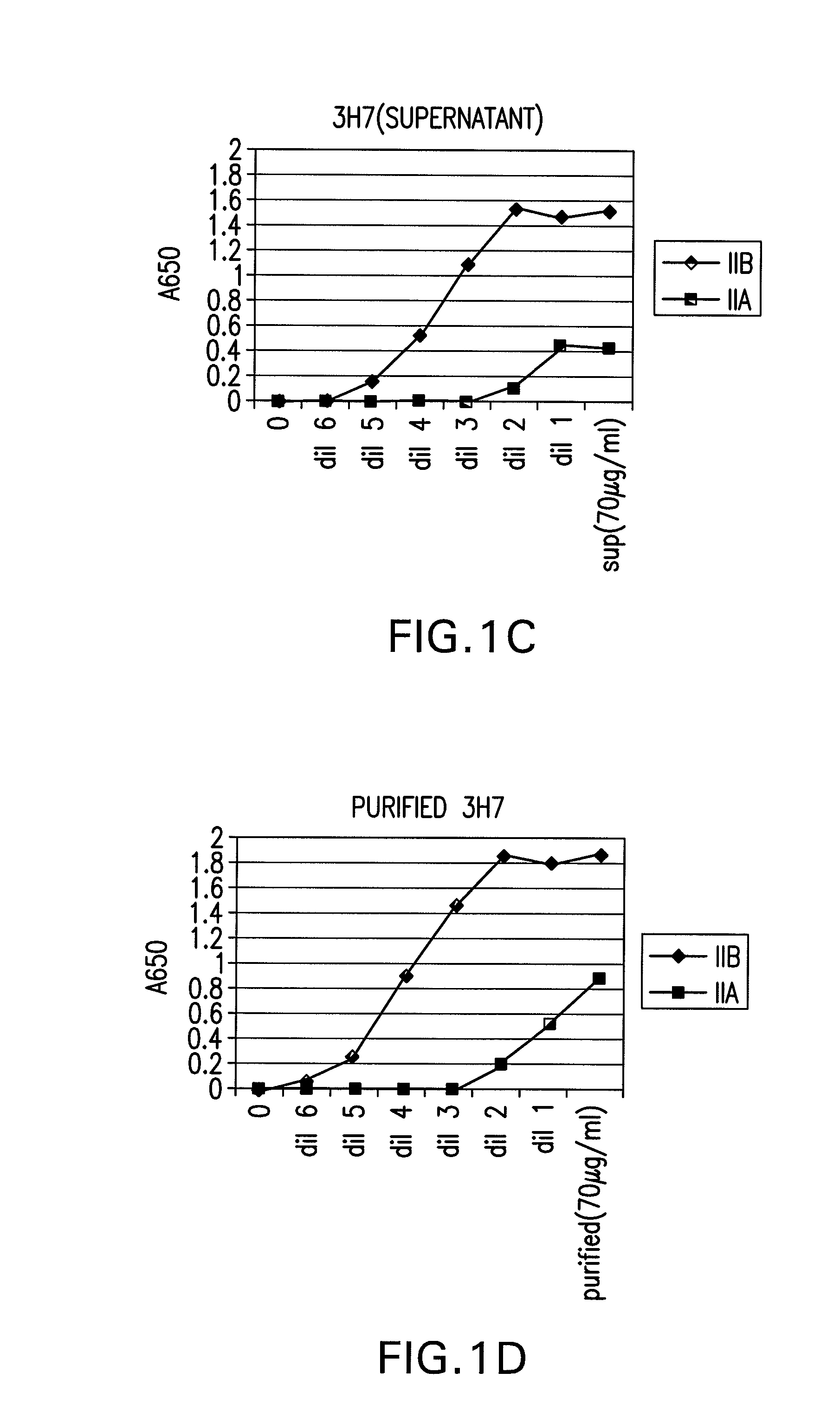
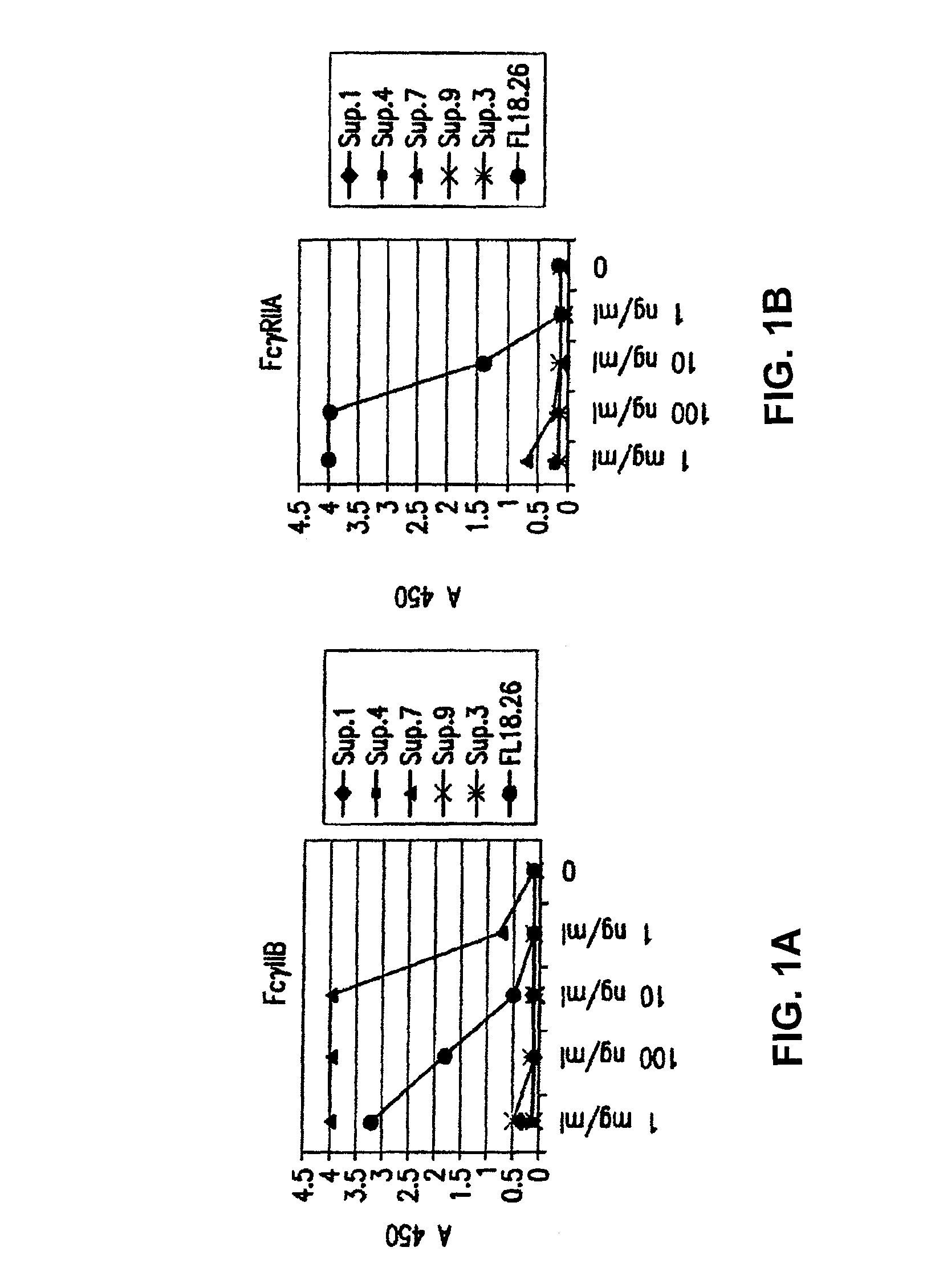
FcgammaRIIB-specific antibodies and methods of use thereof
InactiveUS20060177439A1Good curative effectConvenient treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Amyloid beta-protein 3(pE)-42 antibodies and uses thereof
InactiveUS7122374B1Many symptomReduce riskAnimal cellsImmunoglobulins against animals/humansSide effectAmyloid beta
AβN3pE-42 is a β amyloid protein that accumulates specifically as a major constituent of senile plaque in the brains of both sporadic and familial Alzheimer's disease patients. The invention provides antibodies that specifically recognize AβN3pE-42 and can be expected to have a strong β amyloid-removing action. Particularly, humanized antibodies against AβN3pE-42 are useful to treat human neurodegenerative diseases. Further, since AβN3pE-42 is localized in the brain, the antibodies of the invention can avoid side effects such as kidney disorders caused by the formation of antigen-antibody complex in the blood. An agent for gene therapy using a vector in which a cDNA encoding a protein comprises the antigen-binding region of the antibody can be an efficient therapeutic drug for removing β amyloid from the brain.
Owner:SAIDO TAKAOMI +1
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
FcγRIIB specific antibodies and methods of use thereof
InactiveUS8946387B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090076251A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Mass tags for mass spectrometric analysis of immunoglobulins
InactiveUS20120196297A1Particle separator tubesDepsipeptidesMass spectrometricAntigen-Antibody Complex
The present invention is a method for the characterization or detection of one or more antibodies in a sample. The method comprises obtaining a tagged antigen comprising an antigen and a mass tag attached to the antigen. The tagged antigen has specificity for the antibody. In addition, the method comprises combining the tagged antigen with the antibody to form a tagged antigen-antibody complex. Further, the method comprises cleaving the mass tag from the tagged antigen-antibody complex. Thereafter, the method comprises analyzing the mass tag via a mass spectrometer to determine the presence of the mass tag in the sample and correlating the presence of the mass tag with a presence of the antibody in the sample.
Owner:YOST RICHARD A
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
ActiveUS20090092610A1Enhance immune responseImprove responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
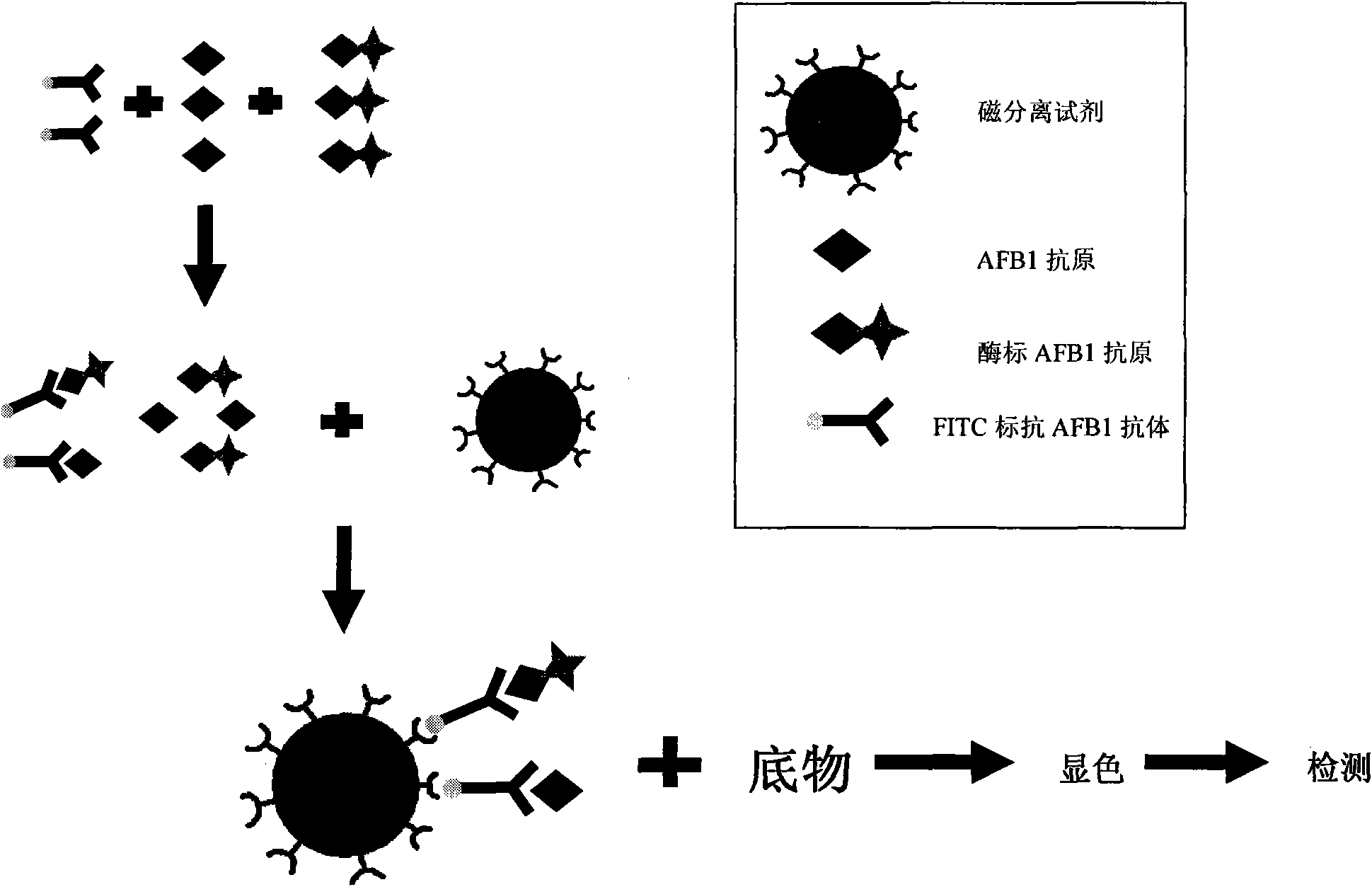
Aflatoxin B1 magnetic particle separation enzyme-linked immunoassay
InactiveCN101661036ASimple processing methodHigh detection sensitivityColor/spectral properties measurementsFluorescence/phosphorescenceSorbentFluorescein isothiocyanate
The invention provides an aflatoxin B1 (AFB1) magnetic separation enzyme-linked immunity quantitative detection method, belonging to the field of food safety immunoassay technique. The method adopts the immuno-detection principle of competition law; and AFB1 is connected with biological enzyme to prepare enzyme-labeled antigen reagent, anti-fluorescein isothiocyanate (FITC) antibody is absorbed onthe surfaces of magnetic particles to prepare magnetic separation reagent, and the FITC is connected with the AFB1 antibody to prepare anti-reagent. In a sample, the AFB1 competes with the enzyme-labeled AFB1 and is combined with a small amount of FITC-labeled anti-AFB1 antibody, so that antigen-antibody complex can be formed. After the magnetic separation reagent is added, the complex is caughtonto the surfaces of the magnetic particles by the anti-FITC antibody connected on the surfaces of the magnetic particles. After being washed, the product is finally added with substrate and detected.The method has the advantages that (1) the magnetic particles are used for replacing the traditional enzyme-labeled plate to be taken as a solid-phase carrier, so that immunoreaction is carried out under the approximate liquid phase condition; and the reaction is more complete and rapid, and has the characteristics of high specificity and good repeatability compared with the traditional enzyme-linked immuno sorbent assay (ELISA); furthermore, (2) by adopting one-step competition law principle, the time used for detection is short.
Owner:北京倍爱康生物技术有限公司
Diagnosis and treatment of malignant neoplasms
InactiveUS20050123545A1Increase stringencyCompound screeningOrganic active ingredientsAbnormal tissue growthMammal
The invention features a method of inhibiting tumor growth and / or tumor invasiveness in a mammal by administering to a mammal a compound (e.g., an antagonistic antibody) which inhibits expression or enzymatic activity of human aspartyl (asparaginyl) beta-hydroxylase (HAAH). The invention also features a method for diagnosing the growth of a malignant neoplasm (e.g., pancreatic cancer) in a mammal by contacting a tissue or bodily fluid from the mammal with an antibody which binds to a HAAH polypeptide under conditions sufficient to form an antigen-antibody complex and / or detecting the antigen-antibody complex.
Owner:WANDS JACK +3
Antigen antibody complex dissociation liquid kit and application thereof
InactiveCN101832999AFacilitate dissociationIncrease the absolute concentration valueBiological testingSerum igeBlood plasma
The invention discloses an antigen antibody complex dissociation liquid kit, which is prepared by blending reagents of 0.3 volume percent of Triton-X100, 1.5 volume percent of lauryl sodium sulfate and 1.5 volume percent of 3-[(3-cholane amidopropyl)-dimethylammonium]propanesulfonic acid in equal amount. The invention also discloses application of the kit in separating a pathogenic antigen antibody complex. The kit of the invention effectively realizes rapid dissociation of the pathogenic antigen antibody complex in a serum or blood plasma sample to be detected, increases the absolute concentration value of an antigen and an antibody, and greatly improves the detection sensitivity of the sample to be detected.
Owner:山东莱博生物科技有限公司
Novel humanized anti-CD22 antibody
ActiveCN103214578AMaintain biological propertiesLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMurine antibodyImmunogenicity
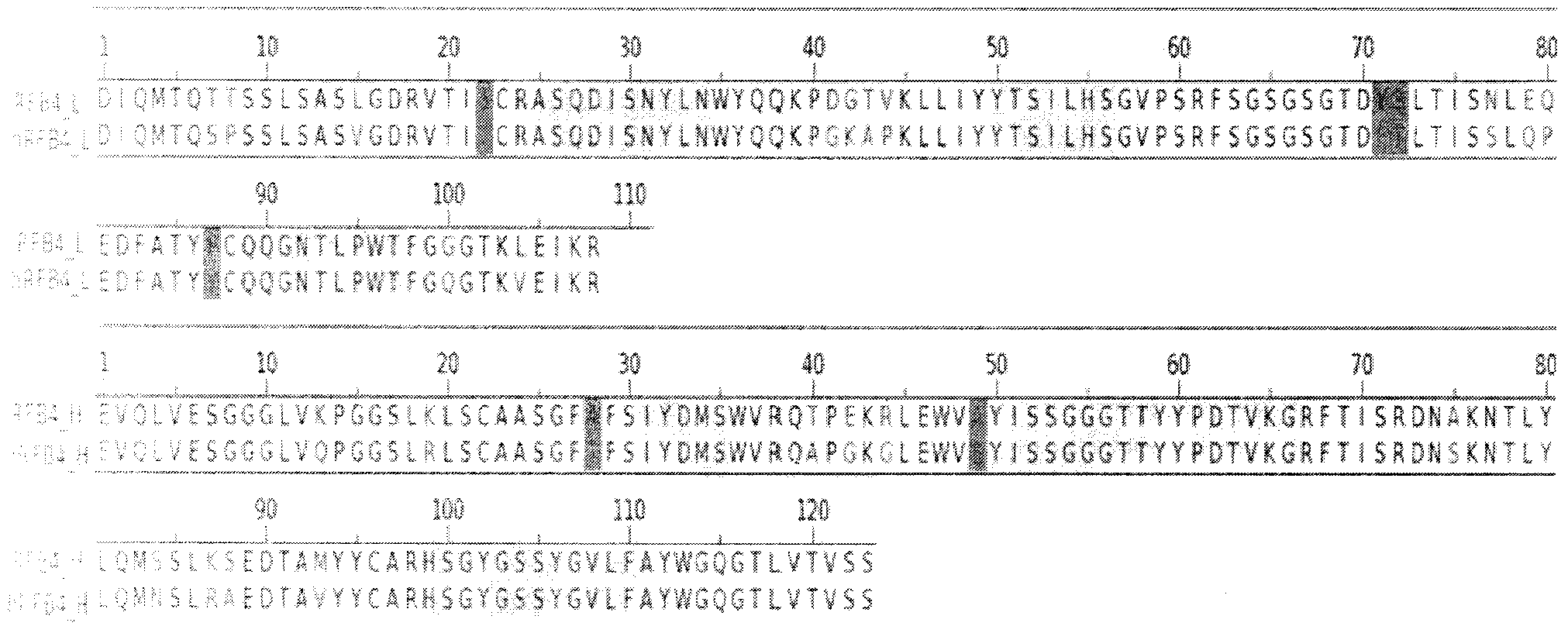
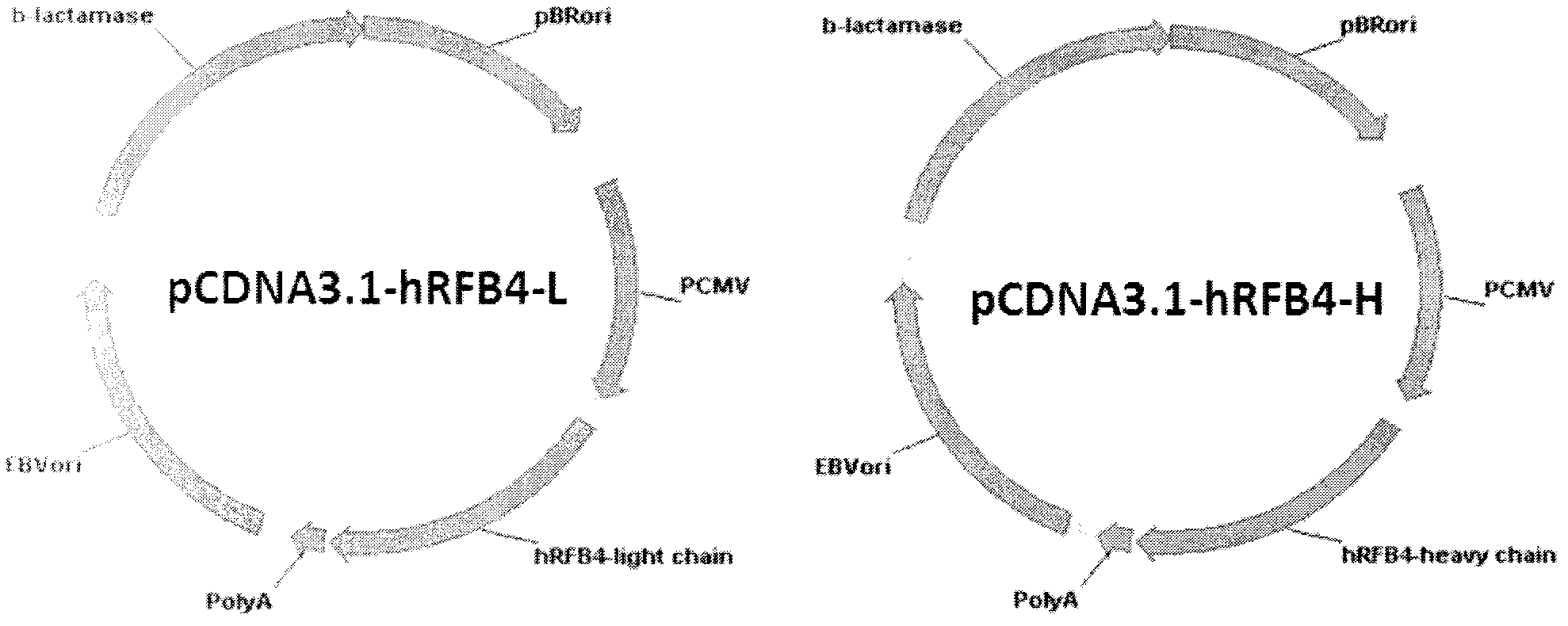
The invention relates to the field of bioengineering, and specifically relates to a novel humanized anti-CD22 antibody. The novel humanized anti-CD22 antibody takes a murine antibody RFB4 as a parental antibody and uses a CDR implantation technique to carry out humanization; and the humanized new antibody hRFB4 has affinity similar with the parental antibody, and keeps the bonding capability of the parental antibody with the CD22 antigen and the cell internalizing activity of antigen-antibody complex caused by the antibody. Compared with the parental antibody, the humanized protein sequence proportion in the humanized hRFB4 antibody reaches over 90%, so that the immunogenicity is greatly reduced and the occurrence of HAMA reaction is avoided.
Owner:BEIJING DONGFANG BIOTECH
Method for detecting third type hepatitis virus antibody by using magnetic micro-particle as transporting species
InactiveCN101551394AThe detection process is fastStrong specificityChemiluminescene/bioluminescenceRadioactive contaminationAntigen-Antibody Complex
The present invention discloses a method for detecting third type hepatitis virus antibody by using magnetic micro-particle as transporting species which includes steps as follows: (1) using magnetic micro-particle as transporting species for reacting and separating, coupling third type hepatitis virus antibody on the magnetic micro-particle surface; (2) adding into confining liquid, oscillating 10 min with middle speed under condition of 20-40 deg c, magnetic separating, giving up supernatant; (3) combining the third type hepatitis virus antibody packed on the magnetic micro-particle surface with the specificity antibody on waited blood; (4) separating the magnetic micro-particle with specificity antigen-antibody complex on surface through an externally-applied magnetic field; (5) combining the antibody complex on the magnetic micro-particle surface with enzyme label rat mouse-anti-body IgG; (6) using chemiluminescence detector, irradiancy substrates A liquor and B liquor for detecting sample irradiancy value. The method has characteristics of rapid detecting speed, high specificity, stabilising, no radioactive pollution which can increase sensitivity and stabilizing ability for detecting.
Owner:AUTOBIO DIAGNOSTICS CO LTD
FcγRIIB specific antibodies and methods of use thereof
ActiveUS8968730B2Enhance immune responseImprove responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
Owner:MACROGENICS INC
Method for detecting protein content difference
InactiveCN101382552AReduce consumptionReduce dosageBiological testingProtein insertionOligonucleotide
The invention discloses a method for determining the differences of protein contents, which comprises the steps that: a. a plurality of kinds of protein antibodies respectively combined with different oligonucleotide sequences synchronously carry out antigen antibody reaction with one kind of proteantigen to be tested; b. the oligonucleotide sequences of the antigen antibody complex in the step a are coupled together through the coupled reaction by using ligase, and the sequences of a ligation product are led to contain a base sequence representing the source of the protein to be tested; c. the ligation product in the step b is taken as a templet, and a base sequence containing the specificity of the source of the protein to be tested is amplified by adopting the amplification reaction; d. the base sequence representing the source of the protein to be tested in the amplification product sequence is determined by adopting the real-time sequencing technology; and e. the sequence determined in the step d is taken as the source of the protein to be tested, signal intensity of each sequence is compared to obtain the relative contents of the protein to be tested in each source. The method can determine the differences of protein expression content from different sources at tone time with low cost.
Owner:周国华
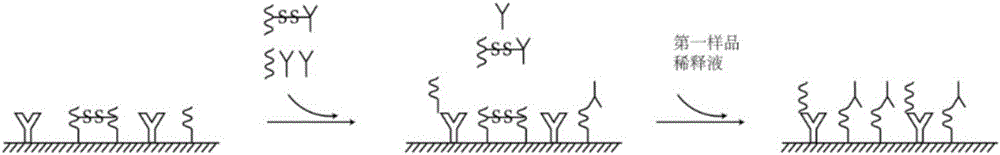
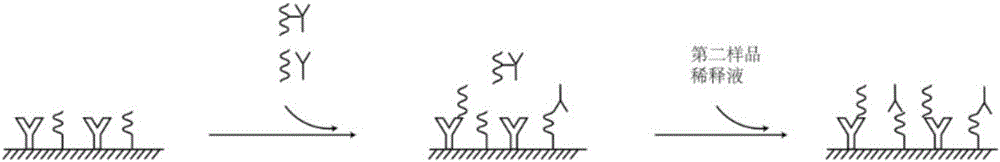
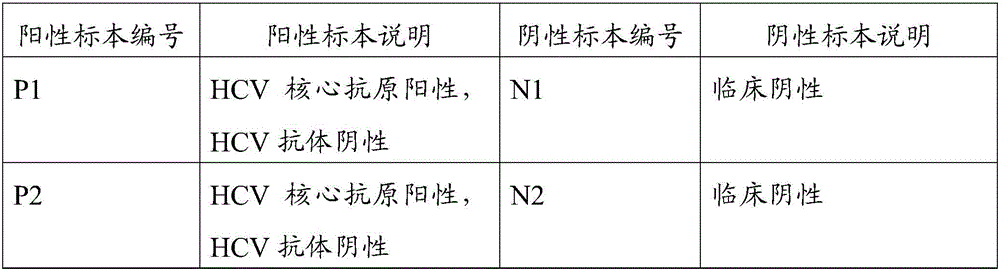
Combined detection kit for hepatitis c virus (HCV) antigen-antibody
InactiveCN106093402AImprove specific recognition abilityReduce non-specific bindingMaterial analysisHCV AntibodyHcv core antigen
The invention discloses a combined detection kit for HCV antigen-antibody. The kit comprises a first monoclonal antibody against HCV core, a first HCV recombinant chimeric antigen, a first diluted sample solution, a second diluted sample solution, a first enzyme-labeled second monoclonal antibody against HCV core antigen, a first ligand-labeled second HCV recombinant chimeric antigen, a second enzyme-labeled second ligand, a developer and a stopping solution, wherein the first diluted sample solution comprises a reducing agent, a first surfactant and a first denaturant; and the second diluted sample solution comprises a second surfactant and a second denaturant. The combined detection kit for the HCV antigen-antibody opens mismatched disulfide bonds in coating antigen and in an antigen-antibody complex of a sample in virtue of the first diluted sample solution, and destroys HCV core antigen and HCV antibody compounds in virtue of the second diluted sample solution, so more antigen is released; and thus, detection sensitivity is improved.
Owner:HUNAN KANGRUN PHARMA
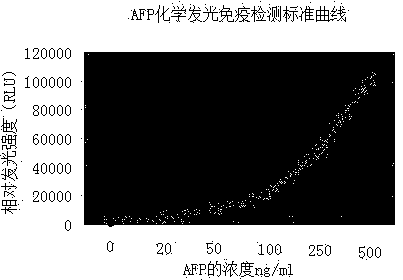
Magnetic particle chemiluminescence immune assay kit of tumor marker AFP (alpha fetal protein) and detection method thereof
InactiveCN104034892AEasy to separateAdequate and prompt responseChemiluminescene/bioluminescenceDisease diagnosisFluorescein isothiocyanateImmuno detection
The invention relates to a magnetic particle chemiluminescence immune assay kit of tumor marker AFP (alpha fetal protein) and a detection method of the magnetic particle chemiluminescence immune assay kit, which belongs to the technical field of the immune detection and analysis. An AFP monoclonal antibody marked by fluorescein isothiocyanate (FITC) and a monoclonal antibody marked by alkaline phosphatase (AP) are combined with an antigen to form a sandwiched immune compound of an FITC marked antibody-antigen-AP marked antibody, and the sandwiched immune compound is similar to a sandwich structure. Then the magnetic particles which are connected with the anti-FITC antibody are added, the specific binding of the anti-FITC antibody and the FITC enables antigen-antibody complex to be connected onto the magnetic particles, the magnetic particles are directly precipitated in an external magnetic field, and the compound formed in the immune reaction manner can be separated from other non-combined substances without centrifuging. According to the kit, the chemiluminescence is combined with the magnetic particles, a reaction system which is approximate to the homogenous phase is provided, and compared with the prior art, the kit has the advantages of high sensitivity, wide linear range, high speed and the like; moreover, the product cost is greatly reduced, and the application prospect on the aspects such as the clinical inspection is promising.
Owner:GUANGXI DOCTOR HAIYI INFORMATION TECH
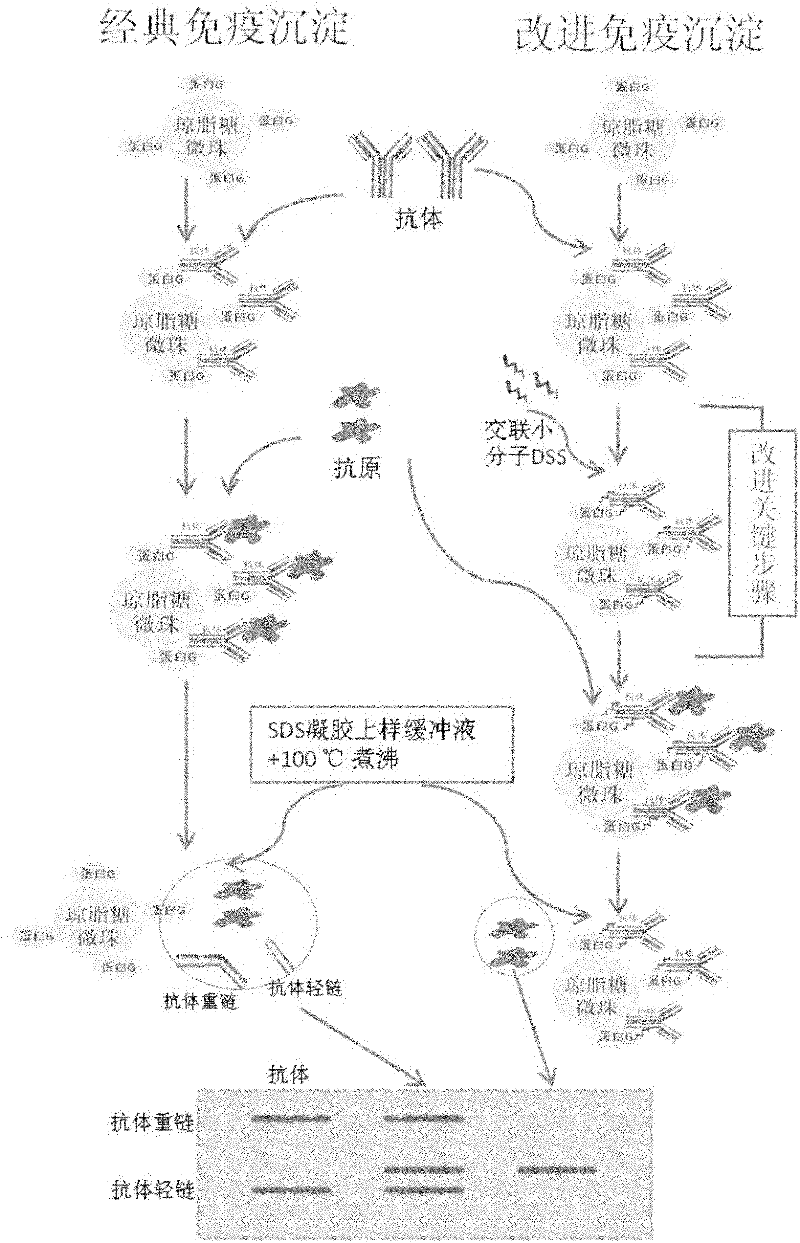
Improved co-immunoprecipitation technical method
The invention belongs to an immunodetection field and specifically relates to an optimized co-immunoprecipitation technology. Based on an original co-immunoprecipitation technology, the co-immunoprecipitation technology is combined with a protein crosslink technology. That is to say, a protein crosslinking agent is used to crosslink an antigen antibody complex; elution is carried out by the use of a specific buffer; and the immunoblotting detection remarkably reduces the pollution of heavy chain and light chain of the antibody in the experiment. According to the invention, specificity of the co-immunoprecipitation method is raised, the method is characterized by stability and repeatability, experiment efficiency is improved, and the method provides beneficial assistance for in-depth research in molecular biology, molecular oncology, cell biology, biochemistry and the like.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
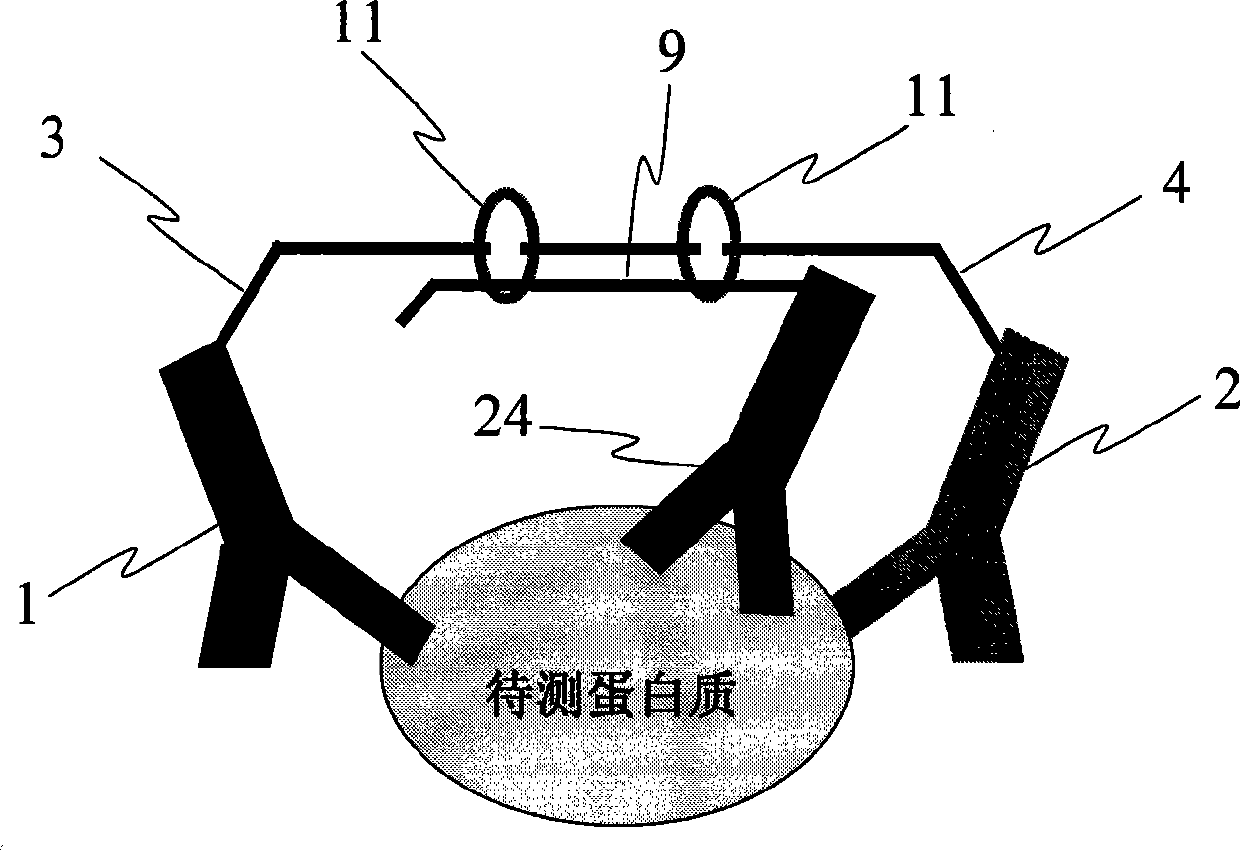
Antigen-antibody complex for preventing and/or treating avian influenza
ActiveCN101732716AChange submission pathEnhance immune functionAntiviralsAntibody ingredientsAvian influenza virusOrganism
The invention provides an antigen-antibody complex for preventing and / or treating avian influenza, which comprises inactivation avian influenza totiviruses which are used as antigen and an immune body thereof, and the mass concentration ratio of the antigen and the immune body is more than 1. After entering organisms to perform initial immunization, the antigen-antibody complex stimulates the organisms again to induce immunoreaction, and immune response is quick in speed and strong in reaction; the antigen-antibody complex used as a carrier is more favorable for capturing and presenting antigen presenting cells and can also strengthen a breeder reaction of T cells effectively; purified totiviruses used as the antigen increase the molecular weight of the complex, are more favorable for ingestion of immunocyte of the organisms on the antigen, cause the more extensive immunoreaction quickly, and have a significance for preventing avian influenza viruses of which the antigen is easy for variation. A preparation of the antigen-antibody complex does not need to use solid carriers, does not cause intense stimulus on the organisms or initiates the organisms to generate an adverse reaction, and is simple to prepare and safe and convenient to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
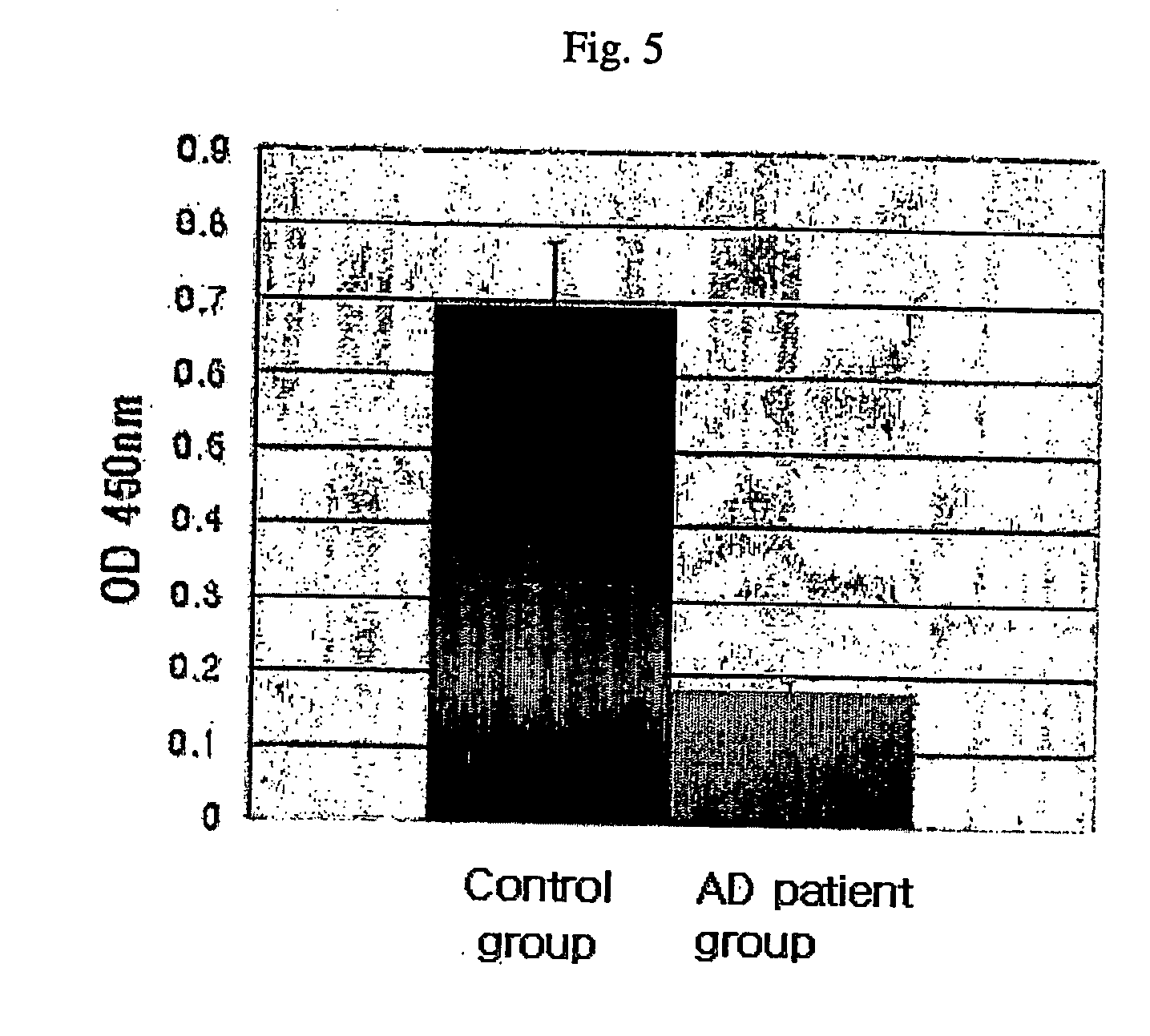
Method for measuring the level of anti-beta-amyloid antibody in body fluids and diagnostic kit for alzheimer's disease using same
InactiveUS20060188951A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDisease patientAmyloid
A method for measuring a concentration of a β-amyloid antibody in a body fluid sample through a reaction forming an antigen-antibody complex uses an antigen protein binding specifically to the β-amyloid antibody existing in a body fluid of an Alzheimer's disease patient. A diagnostic kit for Alzheimer's disease using the method is also provided.
Owner:DIGITAL BIO TECHNOLOGY CO LTD
Kit for determining myoglobin
InactiveCN105911298AHigh detection sensitivityEasy to detectDisease diagnosisBiological testingPolyethylene glycolTurbidity
The invention relates to the technical field of medicine and biochemistry and particularly relates to a kit for determining myoglobin. The kit is composed of double reagents R1 and R2, wherein the reagent R1 is prepared from the following components: 15mmol / L-145mmol / L of a Tris buffering solution, 50mmol / L-200mmol / L of sodium chloride, 5g / L-20g / L of polyethylene glycol-8000, 0.4g / L-1.2g / L of sodium azide, 12g / L-32g / L of bovine serum albumin and a solvent which is a purified water; the reagent R2 is prepared from the following components: 50mmol / L-150mmol / L of a Tris buffering solution, 4g / L-26g / L of bovine serum albumin, 5g / L-15g / L of glycerol, 0.6g / L-1.2g / L of sodium azide, 1g / L-4g / L of an emulsion-coated anti-human myoglobin antibody and a solvent which is purified water. Myoglobin (MYO) in the sample can be combined with the corresponding specific anti-MYO antibody in the reagent R2 to form an antigen-antibody complex and certain turbidity is generated. The turbidity level and the content of an antigen form a direct proportion when the certain antibody exists. The turbidity is determined under a certain wavelength and quantitative determination of the myoglobin can be carried out through a multi-point calibration curve. The kit has the advantages of high accuracy, convenient operation and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
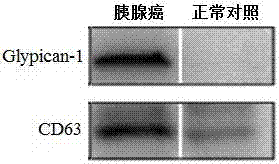
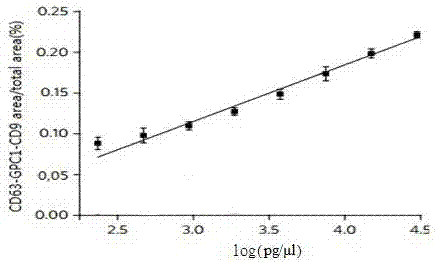
Application of Glypican-1 protein in diagnosis of pancreatic cancer, detection method of positive exosome concentration, and use of detection method
InactiveCN106950374AServe as a diagnosisPlay the role of staging judgment, etc.Raman scatteringBlood plasmaBiology
The invention discloses an application of a Glypican-1 protein in the diagnosis of pancreatic cancer, a detection method of a Glypican-1 positive exosome concentration, and a use of the detection method. A nano-plasma enhanced scattering (nPES) detection technology can be used to quantify exosomes in serum, an exosome extracting process is omitted, the characteristic antigen of the exosomes is used as a target, and the antigen and a corresponding antibody carrying gold nanoparticles form an antigen-antibody complex in order to obtain exosomes, and the amount of the exosomes is reflected by using the light radiation principle of the gold nanoparticles. The Glypican-1 is a pancreatic cancer-derived exosome biomarker, and the content of Glypican-1 positive exosomes in blood plasma has specific specificity even in early pancreatic cancer, so the exosome content index can be used to diagnose the pancreatic cancer, and the exosome level is closely related to the staging and progression of pancreatic cancer patients. Experiments prove that the method using the nPES detection technology to detect the content of the Glypican-1 exosomes in the serum is concise and accurate, and has better sensitivity and specificity than CA19-9 in the diagnosis of the pancreatic cancer.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Process for three-dimensional structural information design new pattern pharmaceutical molecule based on antigen-antibody action
InactiveCN1523350AMicrobiological testing/measurementBiological testingEpitopeExperimental validation
The invention relates to a new-drug molecule designing method based on stereochemical structure information by the action of antigen and antibody. Concretely speaking, it designs theoretical conformation of changeable region of antigen and antibody on the computer, on this basis builds the theoretical conformation of antigen-antibody complex, identifies epitope in antigen combined with antibody and verifies the epitope by experiment, and designs a new-drug molecule according to the sequence of the epitope.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Method for rapidly detecting CP4-EPSPS in transgenic plant
InactiveCN106885907AEasy to manufactureEase of mass productionBiological testingNitrocelluloseCarbon label
The invention relates to a method for rapidly detecting CP4-EPSPS in transgenic plants, comprising the following steps: step 1, preparation of colloidal carbon; step 2, preparation of colloidal carbon-labeled antibodies suitable for pH conditions; step 3, antibody coating to The reaction membrane is used as the T line and the C line of the reaction membrane coated with the secondary antibody; step 4, preparation of colloidal carbon test strips; step 5, extracting protein from the plant to be tested, and preparing protein extract; step 6, testing the test strip The paper strip is inserted into the protein extract solution to react; Step 7, after an appropriate reaction time, judge the result with the naked eye. The present invention adopts the colloidal carbon pad labeled with polyclonal antibody E01, the nitrocellulose membrane coated with polyclonal antibody E01 and the nitrocellulose membrane coated with goat anti-rabbit IgG to make detection test strips; The principle of immunochromatography is to form antigen-antibody complexes to make the results visible to the naked eye.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI +1
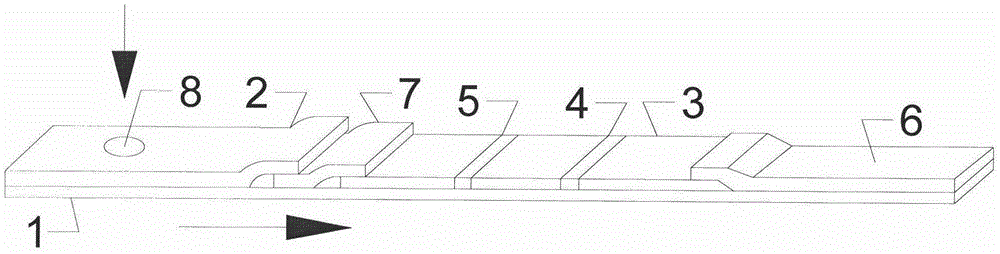
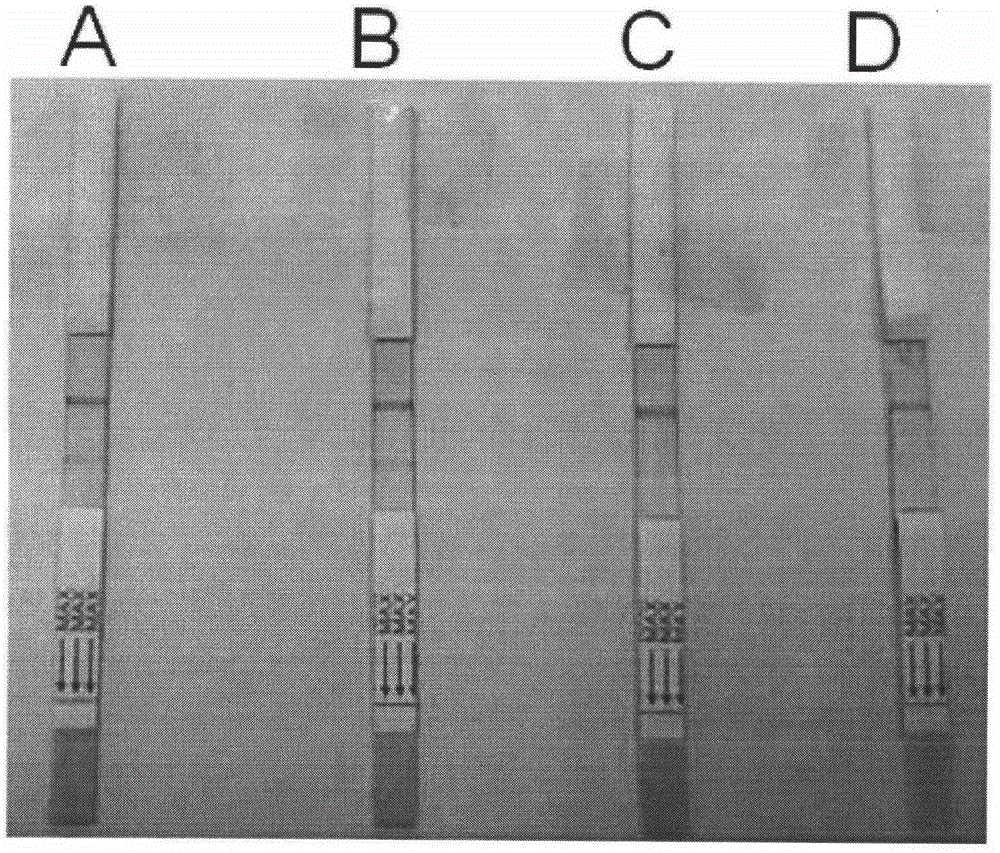
Colloidal gold test strip for quick diagnosis of total swine HEV (Hepatitis E Virus) antibody
ActiveCN103823057AEpidemic Prevention and ControlEasy diagnosticsMaterial analysisCelluloseBinding site
The invention discloses a colloidal gold test strip for quick diagnosis of a total swine HEV (Hepatitis E Virus) antibody, and a preparation method of the colloidal gold test strip. The colloidal gold test strip comprises a water-absorbing glass fiber layer, a cellulose nitrate membrane layer, a water-absorbing filter paper layer and a white plastic backing plate. The preparation method comprises the following steps: preparing an HEV coating antigen, an HEV labeled antigen and an HEV labeled antigen-colloidal gold, and assembling the colloidal-gold quick diagnosis test strip. The test strip can be used for detecting the total swine hepatitis E virus antibody, is simple in diagnosis operation and short in detection time and portable, provides a corresponding research basis and technical guarantee for large-scale survey of the HEV eqidemiology, adopts a double antigen sandwich method principle to enable the coating antigen and an antibody in blood serum to generate specific binding and the antigen and antibody reaction to be regenerated by combining with an antigen-antibody complex and a labeled antigen so as to allow the two antigens to bind different antigen binding sites, and is good in specificity and high in sensitivity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
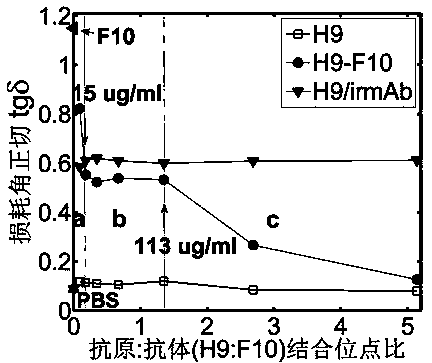
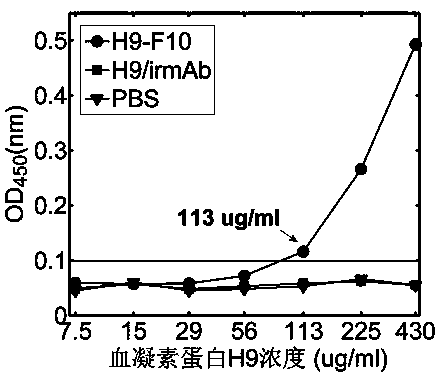
Terahertz time-domain spectroscopy-based unmarked hemagglutinin detection method
ActiveCN104215776AHigh detection sensitivityImprove detection efficiencyBiological testingHemagglutininTime domain
The invention discloses a terahertz time-domain spectroscopy-based unmarked hemagglutinin detection method which comprises the following steps: a. dissolving a sample into a PBS buffer solution to prepare an antigen solution H9 with different concentration, and reacting a monoclonal antibody F10 with the antigen solution H9 according to an equal volume ratio to obtain a reactant; b. incubating the reactant at room temperature for a certain period of time, then shaking, and staying overnight under a low-temperature condition, thereby forming an antigen-antibody complex structure; c. placing the complex into a detection box, vertically placing the detection box at the focal spot of a terahertz light beam, and recording concentration-related terahertz intensity absorption spectrum; and d. according to the concentration-related terahertz intensity absorption spectrum, judging whether an absorption peak exists, and if not, detecting hemagglutinin in the sample.
Owner:SHENZHEN UNIV
Method for rapidly preparing chromatin immunoprecipitation sample
ActiveCN106801096AShorten the timeHigh sensitivityMicrobiological testing/measurementMagnetic beadA-DNA
The invention discloses a method for rapidly preparing a chromatin immunoprecipitation sample. The method comprises the following steps: performing formaldehyde crosslinking on a cell to fix a complex of a protein and a nucleic acid, extracting the complex of the protein and the nucleic acid, and breaking a chromatin segment into small segments in an ultrasonic or enzymatic hydrolysis manner; suspending an antibody onto a solid-phase support, i.e. a magnetic bead, adding the obtained small segments onto the prepared magnetic bead, performing incubation to obtain a magnetic bead antigen antibody complex, washing an uncombined substance away after incubation is finished, and resuspending the magnetic bead antigen antibody complex to obtain a magnetic bead antigen antibody complex sample to be detected. The method is easy to operate; the prepared magnetic bead antigen antibody complex sample can be directly used for PCR detection or amplification and construction of a DNA library, so that the sample preparation time is obviously shortened, meanwhile, the shortcoming of weaker enrichment signals of certain proteins and DNAs caused by excessive elution steps is overcome, the detection sensitivity is greatly improved, and influence on subsequent PCR identification and DNA segment amplification is avoided.
Owner:WUHAN UNIV
Method for detecting antique silk fabric
The invention discloses a method for detecting antique silk fabric. The method comprises the steps that an indirect ELISA method is used for detecting the silk peptide component of the silk fabric, namely silk fabric antique eluant wraps an elisa plate, and then rabbit anti silk fibroin antibodies are added to be combined with antigens to form an antigen-antibody compound; after washing, alkaline phosphatase enzyme label IgG(H+L) antibodies are added, an antibody-antigen-antibody compound is formed, a tetramethyl benzidine solution is added after washing, a substrate is catalyzed to be a colored product by enzymes, and qualitative analysis can be conducted according to the depth of color variation of the product. Due to the fact that the catalytic efficiency of the enzymes is very high, the immunoreaction result is indirectly acquired, and the test has high sensitivity.
Owner:ZHEJIANG SCI-TECH UNIV
Method for preparing warm start Taq enzyme
InactiveCN107119029AStrong specificityImprove reliabilityTransferasesImmunoglobulins against enzymesEnzyme immunoassaysAntigen-antibody reactions
The invention discloses a real-time warm start Taq enzyme and a preparation method thereof. The real-time warm start Taq enzyme is an antigen-antibody complex of a Taq enzyme and a Taq enzyme nano antibody through antigen-antibody reaction, wherein the Taq enzyme nano antibody is derived from but not limited by animals which can generate nano antibodies through Taq enzyme immunoassay. By forming the antigen-antibody complex, the activity of the Taq enzyme can be released only at relatively high temperature, warm start can be achieved, the specificity, the reliability and the sensitivity of the Taq enzyme are improved, generation of non-specific amplification is avoided, and the Taq enzyme is capable of reducing clone mispairing to a very large extent and achieving efficient specific amplification when being compared with similar products. The nano antibody has high affinity and high stability, is capable of greatly improving specificity, sensitivity and amplification efficiency of warm start PCR (Polymerase Chain Reaction), and is applicable to various PCR reactions.
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
Method for detecting collagen in ancient cultural relic material
ActiveCN103575908ALess destructiveKeep the original appearanceBiological material analysisBiological testingPolyclonal antibodiesQualitative analysis
The invention discloses a method for detecting collagen in an ancient cultural relic material. The method is characterized by comprising the following steps: detecting protein components in the ancient cultural relic material through a competitive enzyme-linked immunosorbent assay method, namely, fixing an AbCam rabbit anti-I type collagen polyclonal antibody on a solid phase carrier, and adding a sample eluent to form an antigen-antibody complex; washing through a phosphate buffer solution (PBS), and adding alkaline phosphatase-labeled goat anti-rabbit IgG(H+L) which is then bonded to the solid phase carrier through reaction, wherein the amount of enzyme on a solid phase is in a certain proportion to the quantity of detected substances in a sample; after a substrate of enzyme reaction is added, catalyzing the substrate into colored products through enzyme, wherein the quantity of the products is positively correlated to the quantity of the detected substances in the sample, so that qualitative analysis can be performed according to the color depth. The method disclosed by the invention is low in cost, simple in detection and high in response speed.
Owner:ZHEJIANG SCI-TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com