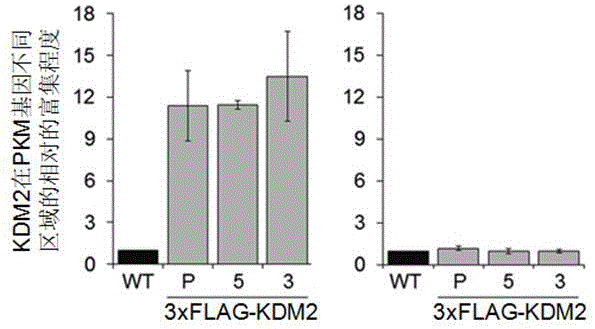
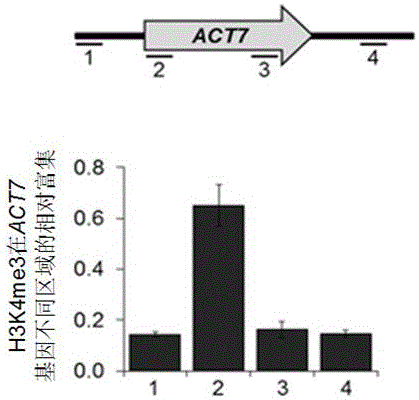
Method for rapidly preparing chromatin immunoprecipitation sample
A co-immunoprecipitation and chromatin technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of high cost, low sensitivity, long time consumption, etc., to save time, improve sensitivity, and elution method simple effect
Active Publication Date: 2017-06-06
WUHAN UNIV
View PDF0 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0008] The preparation results of chromatin immunoprecipitation samples play an important role in subsequent sequencing analysis, and its preparation efficiency also directly affects the time of the entire ChIP-Seq operation. However, the existing sample preparation process is often time-consuming and costly. The detection sensitivity is also low
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0031] This example provides a rapid preparation method for chromatin immunoprecipitation (ChIP) samples.
[0032] Cell material: cultured 293T cells; antibody material: histone H3 antibody, H3K4me3 antibody or Flag antibody;
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for rapidly preparing a chromatin immunoprecipitation sample. The method comprises the following steps: performing formaldehyde crosslinking on a cell to fix a complex of a protein and a nucleic acid, extracting the complex of the protein and the nucleic acid, and breaking a chromatin segment into small segments in an ultrasonic or enzymatic hydrolysis manner; suspending an antibody onto a solid-phase support, i.e. a magnetic bead, adding the obtained small segments onto the prepared magnetic bead, performing incubation to obtain a magnetic bead antigen antibody complex, washing an uncombined substance away after incubation is finished, and resuspending the magnetic bead antigen antibody complex to obtain a magnetic bead antigen antibody complex sample to be detected. The method is easy to operate; the prepared magnetic bead antigen antibody complex sample can be directly used for PCR detection or amplification and construction of a DNA library, so that the sample preparation time is obviously shortened, meanwhile, the shortcoming of weaker enrichment signals of certain proteins and DNAs caused by excessive elution steps is overcome, the detection sensitivity is greatly improved, and influence on subsequent PCR identification and DNA segment amplification is avoided.
Description
technical field [0001] The invention belongs to the technical field of chromatin immunoprecipitation (ChIP), and more specifically relates to a rapid preparation method for chromatin immunoprecipitation (ChIP) samples. Background technique [0002] Histone is a basic protein in the chromatin of eukaryotic cells. It contains a lot of basic amino acids such as arginine and lysine, which add up to about 1 / 4 of all amino acid residues. Histones bind to the negatively charged double-helix DNA to form a DNA-histone complex. Histones are the basic structural proteins of eukaryotic chromosomes. They are a type of small molecular basic proteins. There are five types: H1, H2A, H2B, H3 and H4 histones. They are rich in positively charged basic amino acids and can Interacts with negatively charged phosphate groups in DNA. [0003] Various post-translational modifications (PTMs) (i.e., methylation, acetylation, ubiquitination, and sumoylation) of many histones occur at lysine sites. A...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12Q1/68
CPCC12Q1/6806C12Q2523/308
Inventor 杜海宁李锋王洪岩郑亮德舒文杰洪斌李俊峰
Owner WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com