Novel humanized anti-CD22 antibody
An antibody, human-derived technology, applied in the field of bioengineering, can solve the problems of increasing antibody affinity and efficacy, and achieve the effect of reducing immunogenicity and avoiding HAMA reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, humanization of RFB4 antibody
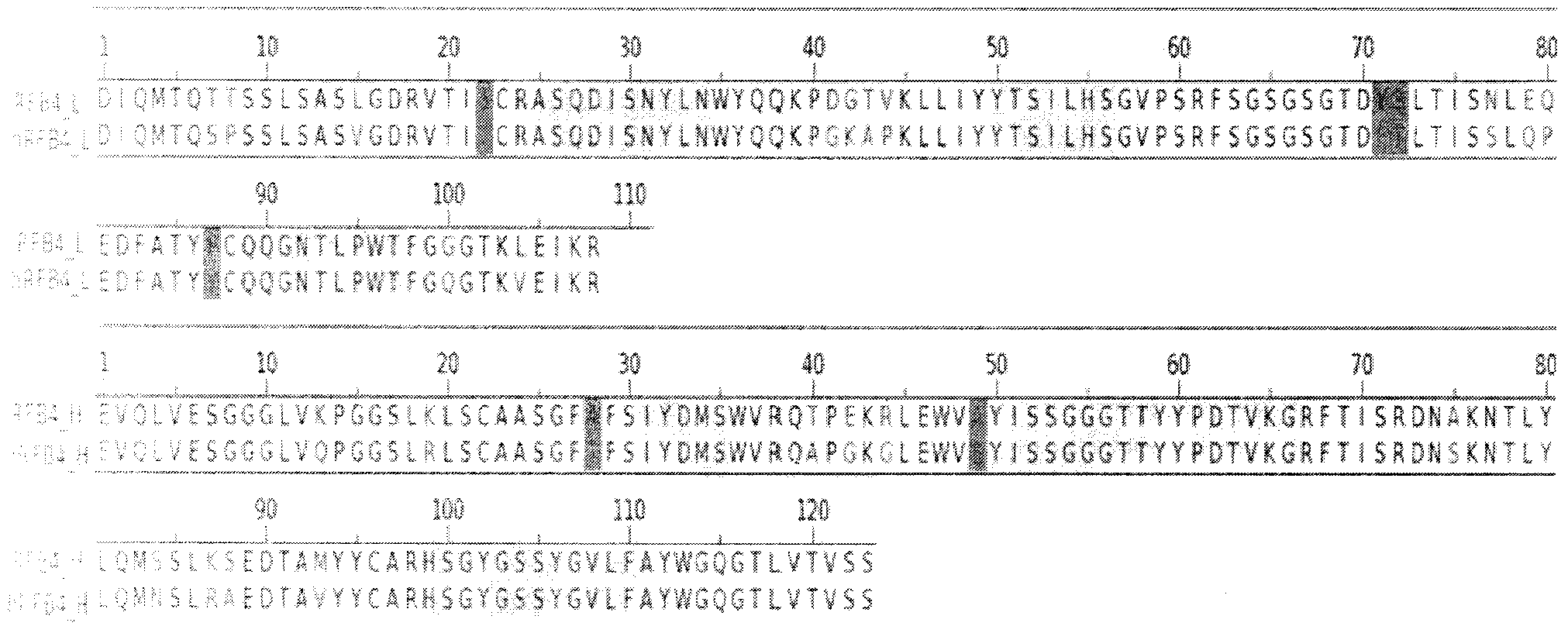
[0049] The light and heavy chain genes of RFB4 are from NCBI, and the nucleic acid codes are: CS110728 and CS110730, respectively. After translating the DNA sequence into an amino acid sequence, the framework region (FR) and complementarity determining region (CDR) of the RFB4 variable region gene were analyzed according to the Kabat rules and with reference to relevant literature and patents.
[0050] Select the human antibody sequences Consensus KI and Consensus subIII with the highest homology to the framework region of the RFB4 mouse sequence in the Kabat database as the framework regions of the light chain and heavy chain of the humanized antibody, and connect the human framework region and the mouse CDR region Finally, as the initial humanized antibody hRFB4 sequence, the specific alignment results are as follows figure 1 shown.
[0051] Using the BLAST method and using PDB as the search database, the homology analys...
Embodiment 2
[0053] Embodiment 2, construction of YQ22-1 light and heavy chain expression vector
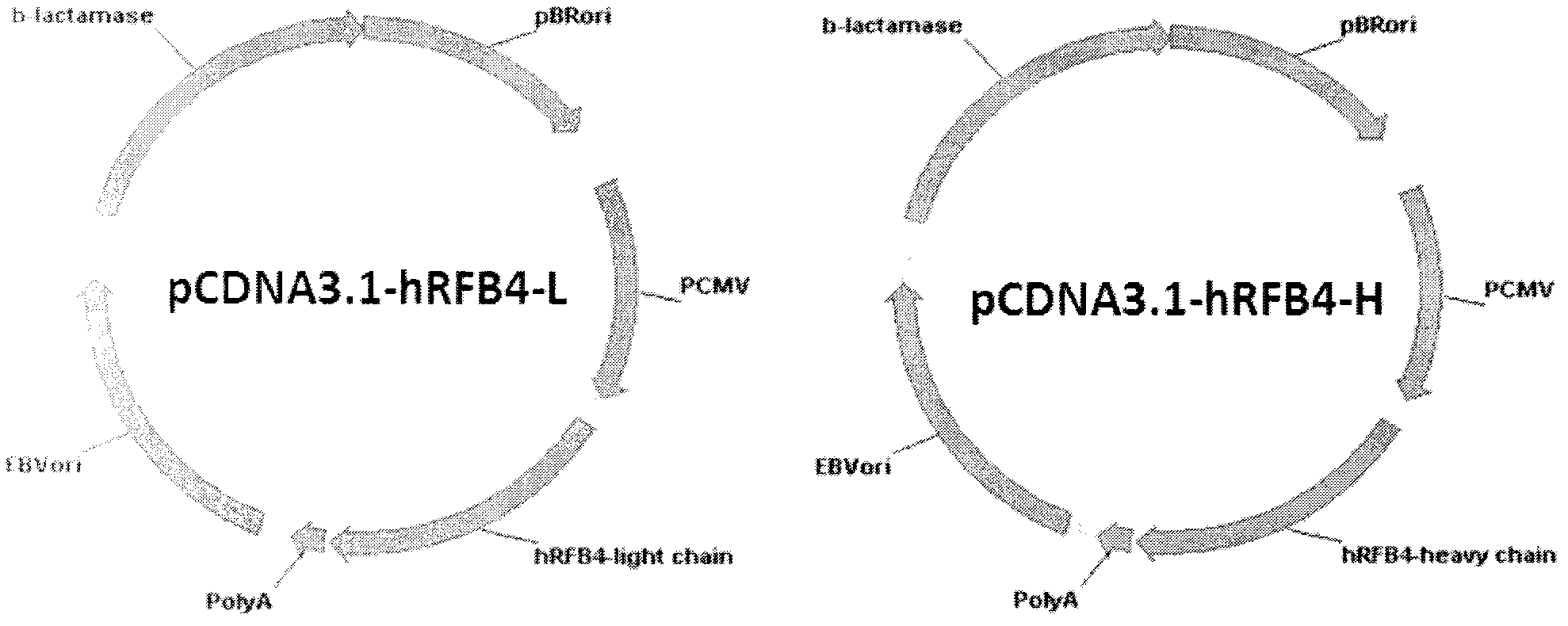
[0054] Entrust Shanghai Jierui Bioengineering Co., Ltd. to synthesize the variable region DNA sequences of YQ22-1 light chain and heavy chain. PCR primers were designed to amplify the antibody light chain and heavy chain genes respectively, and then they were subcloned into the expression vector pCDNA3.1 (Invitrogen Company) with EcoRI, PmlI and SalI, PmlI double digestion respectively,
[0055] Large-scale plasmids are prepared to transfect cells to prepare humanized antibodies. Its plasmid map is as image 3 shown.
Embodiment 3
[0056] Example 3, Expression and Purification of YQ22-1 Antibody
[0057] 1. Co-transfection of YQ22-1 light and heavy chain genes
[0058] The day before transfection, count and observe the cell viability with 6~7×10 5 293E cells were inoculated into 28ml FreeStyle 293 Expression Medium at a cell density of / ml. On the second day, the cell aggregates were shaken to break up, and samples were taken and added to trypan blue to count and observe the cell activity. The number of living cells accounted for more than 90%, and the transfection was carried out. Mix 30μg plasmid with 1ml Opti-MEMI, meanwhile, add 60μl Invitrogen 293 Fectin TM Mix reagent with 1ml Opti-MEMI. Stand at room temperature for 5 minutes. Then add the Opti-MEMI mixed with the plasmid to the Opti-MEMI mixed with the transfection reagent, and mix well to obtain a total of 2ml of the mixed solution. Let stand at room temperature for 20 minutes. At the same time, the 3×10 7 Place 2 cells in 28ml FreeStyle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com