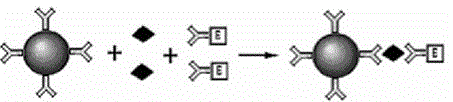
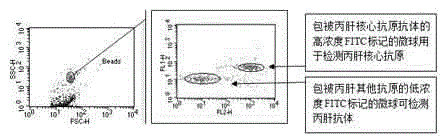
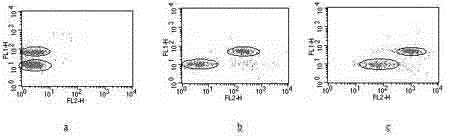
Reagent for high throughput combined detection of hepatitis c virus antigen-antibody
A hepatitis C virus, combined detection technology, applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of missed diagnosis, strong technical ability, complicated method operation, etc., to achieve the effect of enhanced sensitivity and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The components and concentrations of the detection reagents are
[0070] Component 1 (R1) FITC fluorescent microsphere reagent:
[0071] FITC Highlight Microspheres Coated with HCV Antibody 0.1%
[0072] FITC low brightness microspheres coated with HCV protein 0.1%
[0073] Tris 12.1g / L
[0074] BSA 0.5g / L
[0075] PC-300 0.1%
[0076] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0077] Component 2 (R2) PE labeling reagent:
[0078] PE-labeled HCV-cAg antibody 0.1%
[0079] PE-labeled NS3 protein 0.1%
[0080] PE-labeled NS4 protein 0.1%
[0081] PE-labeled NS5 protein 0.1%
[0082] Tris 12.1g / L
[0083] BSA 0.5g / L
[0084] PC-300 0.1%
[0085] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0086] Cleaning fluid:
[0087] Disodium hydrogen phosphate 1.974g / L
[0088] Potassium dihydrogen phosphate 0.224g / L
[0089] Sodium chloride 0.9g / L
[0090] Tween-20 0.5%
[0091] All components were dissolved in deioni...
Embodiment 2
[0113] The components and concentrations of the detection reagents were
[0114] Component 1 (R1) FITC fluorescent microsphere reagent:
[0115] FITC highlight microspheres coated with HCV core antigen antibody 0.5%
[0116] FITC low brightness microspheres coated with HCV protein 0.5%
[0117] Tris 12.1g / L
[0118] BSA 0.5g / L
[0119] PC-300 0.1%
[0120] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0121] Component 2 (R2) PE labeling reagent:
[0122] PE-labeled HCV-cAg antibody 0.5%
[0123] PE-labeled NS3 protein 0.5%
[0124] PE-labeled NS4 protein 0.5%
[0125] PE-labeled NS5 protein 0.5%
[0126] Tris 12.1g / L
[0127] BSA 0.5g / L
[0128] PC-300 0.1%
[0129] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0130] Cleaning solution, positive control, negative control and detection method are as in Example 1.
Embodiment 3
[0132] The components and concentrations of the detection reagents were
[0133] Component 1 (R1) FITC fluorescent microsphere reagent:
[0134] FITC Highlight Microspheres Coated with Hepatitis C Core Antibody 1%
[0135] FITC low brightness microspheres coated with HCV protein 1%
[0136] Tris 12.1g / L
[0137] BSA 0.5g / L
[0138] PC-300 0.1%
[0139] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0140] Component 2 (R2) PE labeling reagent:
[0141] PE-labeled HCV-cAg antibody 1%
[0142] PE-labeled NS3 protein 1%
[0143] PE-labeled NS4 protein 1%
[0144] PE-labeled NS5 protein 1%
[0145] Tris 12.1g / L
[0146] BSA 0.5g / L
[0147] PC-300 0.1%
[0148] Prepare with deionized water and adjust the pH to 7.6 with HCl.
[0149] Cleaning solution, positive control, negative control and detection method are as in Example 1.
[0150] Effect test 1:
[0151] Analyze the accuracy of the kit for combined detection of hepatitis C virus antigen-an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com