A pair of monoclonal antibodies capable of specifically recognizing hcv NS3 protein and its application
A monoclonal antibody, specific technology, applied in the field of molecular biology and immunology, to achieve great application prospects and promotion value, high sensitivity and specificity, and reduce the effect of missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of HCV NS3 monoclonal antibody
[0028] 1.1 Antigen preparation
[0029] According to the HCV NS3 protein sequence (ID: O92530.3) on NCBI GenBank, TMHMM software was used to analyze the transmembrane domain of NS3 protein, and IEDB software was used to analyze the immunogenicity, hydrophobicity and surface accessibility of NS3 protein, and found that NS3 protein 226- The 480aa amino acid has strong antigenicity, hydrophilicity and surface accessibility, and can change the polarity of the corresponding sequence through amino acid mutation. After the above analysis, the final screening determined that the recombinant HCV NS3 antigen polypeptide sequence is: 226-480aa (the amino acid sequence is shown in SEQID NO.9). The short peptide and the N-terminal coupled human IL-1β protein short peptide (IL-1β-NS3) were synthesized by Jinan Boshang Biotechnology Co., Ltd.
[0030] 1.2 Animal immunity
[0031] Take the above-mentioned synthetic antigen, ...
Embodiment 2
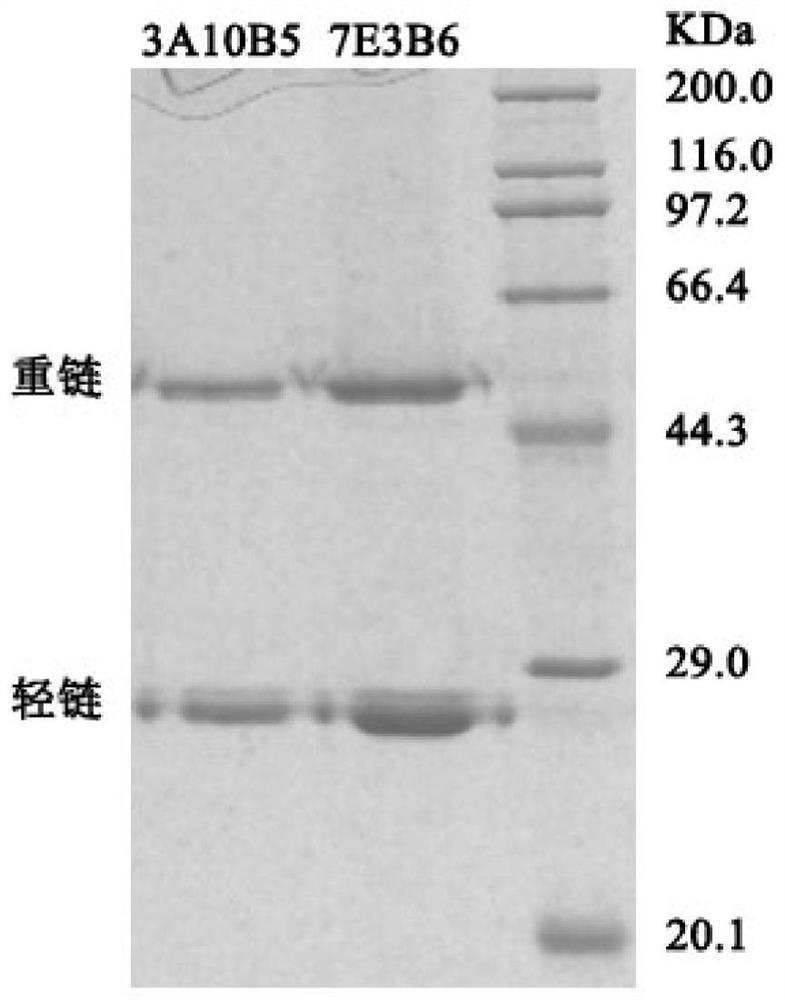
[0048] Example 2: Purification of monoclonal antibodies
[0049] Wash the column with ultrapure water for about 5 column volumes; wash the column with equilibrium solution for about 5 column volumes (flow rate is 1.5ml / min). The crude antibody extract was diluted 2-3 times with buffer, filtered through a 0.45 μm microporous membrane, and then combined with protein A in the filler (1.5ml / min). Rinse the column with equilibration solution until the baseline is equilibrated, at least 10 column volumes. Use the eluent for antibody elution (flow rate 1.5ml / min). When the UV value is higher than 0.1, start to collect the flow-through liquid. When the UV value rises above 2.0, it starts to fall. When the UV value is lower than 0.1, stop receiving flow. The collected flow-through solution is the truncated HCV NS3 monoclonal antibody solution. Concentrate the collected solution to 15 ml using an ultrafiltration concentrator tube. The eluent continues to wash the affinity column unti...
Embodiment 3
[0052] Example 3: Antibody identification
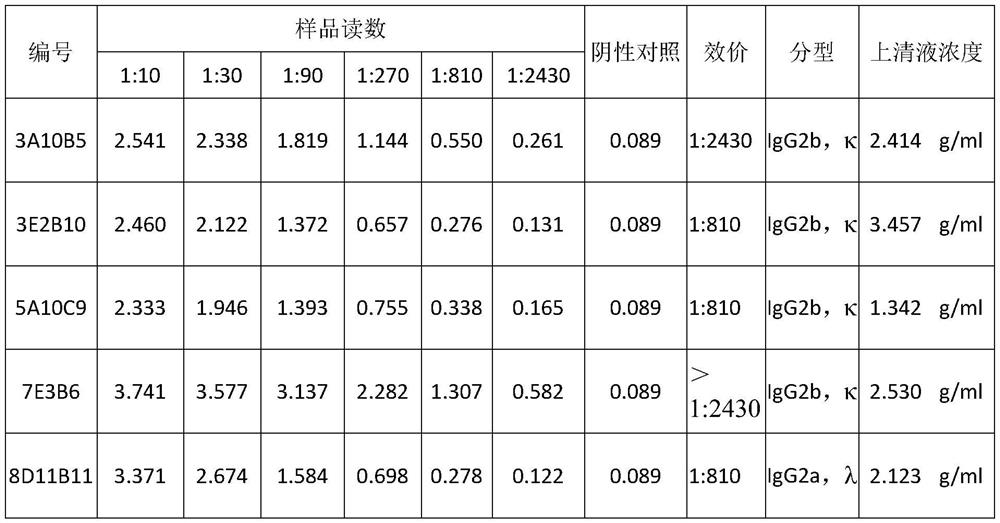
[0053] 3.1 Potency identification of monoclonal antibodies
[0054] The synthetic HCV NS3 antigen short peptide was taken to coat the microtiter plate, and the titer of the purified monoclonal antibody was detected by indirect ELASA after blocking with 5% BSA solution. The ascitic fluid samples containing the above monoclonal antibodies were serially diluted, and PBS was used as a negative control, 100 μl per well, added to the microtiter plate, and incubated at 37°C for 1 hour. After washing with TBST, add 1:4000 diluted HRP-labeled secondary antibody, 100 μl per well, and incubate at 37°C for 1 hour. After washing with TBST, TMB substrate was added to develop color for 20 min, and then the reaction was terminated. Microplate reader reads OD 450 values, and the test results are shown in Table 1.
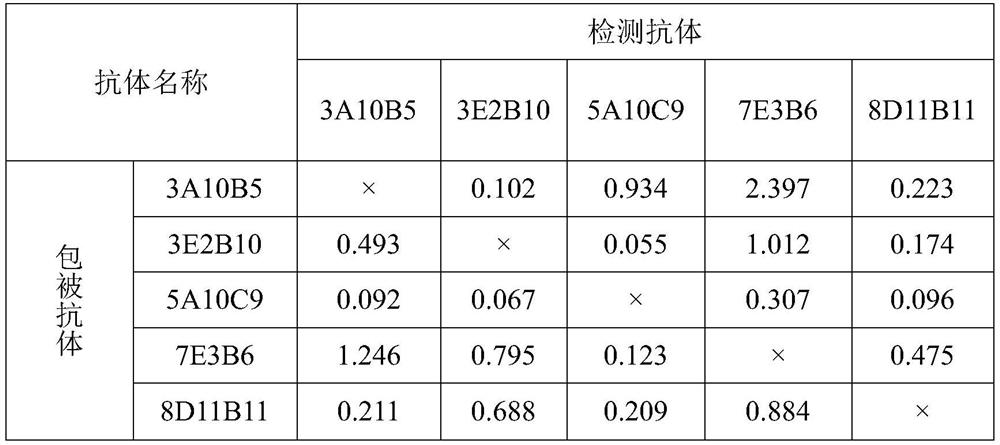
[0055] 3.2 Subtype identification
[0056] Use the antibody typing kit to identify the subtype of the monoclonal antibody, in which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com