Antibody to hepatitis C virus time resolution detection kit and preparation method thereof
A hepatitis C virus and detection kit technology, applied in the field of viral protein immunoassay, can solve the problems of narrow detection range, decreased antigen activity, low sensitivity, etc., and achieve the effects of reduced detection cost, improved sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
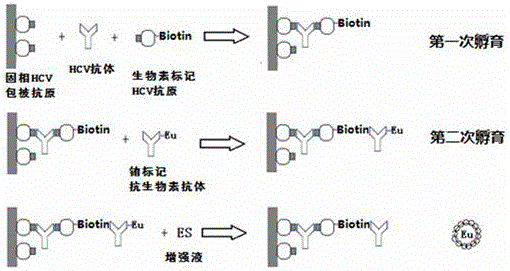
[0028] Example 1 Preparation of Hepatitis C Virus Antibody Time-Resolved Detection Kit
[0029] Use Eu 3+ As a fluorescent marker preparation kit, the specific operation is as follows:
[0030] 1. Obtaining HCV antigen-coated plates:
[0031] Dilute the HCV gene recombinant antigen in an appropriate ratio to 1-3 μg / mL with carbonate buffer solution, add it into the microwell reaction plate, 100 μL / well, coat overnight, wash, block, dry, etc. The orifice strips are vacuum-sealed in aluminum foil bags and kept refrigerated for later use.
[0032]2. Obtaining biotin-labeled HCV antigen: After dialysis of HCV-labeled antigen II and HCV-labeled antigen III through phosphate buffer, according to the ratio (mass ratio of biotin to labeled antigen 1 is 1:3; biotin to labeled antigen 2 with a mass ratio of 1:5) were marked separately, mixed for 10 minutes after marking, and left to stand for about 2 hours. The labeled antigen was dialyzed in phosphate buffered saline for 48 hours (...
Embodiment 2
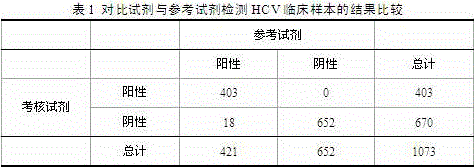
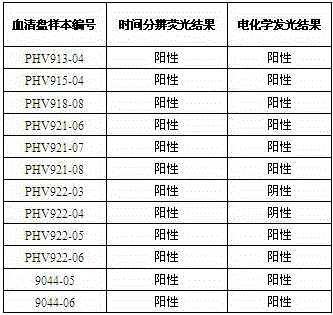
[0037] Embodiment 2 Comparative experiment between the kit of the present invention and the imported luminescence kit
[0038] Utilize the hepatitis C virus antibody detection kit (time-resolved immunofluorescence method) of the present invention and the hepatitis C virus antibody detection kit (electrochemiluminescence method) Anti-HCV II of Germany RocheDiagnostics GmbH company to detect 1073 routine serum samples simultaneously, Including 422 cases of HCV-positive serum samples and 651 cases of HCV-negative serum samples (including 60 cases of interference samples, interference factors include CMV / Rubella / ANA / HBsAg / RF / HSV / TP / HCG). In addition, 111 of the 422 positive serum samples corresponded to homologous plasma samples, 115 of the 651 negative serum samples corresponded to homologous plasma samples, and 12 samples of BBI / Zepto serum conversion discs were tested. If the results of the initial test of the sample are inconsistent, use the two test kits to test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com