A hepatitis C virus antibody detection reagent comprising recombinant fusion antigen a and b and its application and recombinant fusion antigen a and b
A hepatitis C virus and fusion antigen technology, applied in the field of bioengineering, can solve the problems of poor antigen stability, incomparable reagent performance, incorrect folding, etc., to reduce the risk of sample missed detection, optimize clinical detection effect, The effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
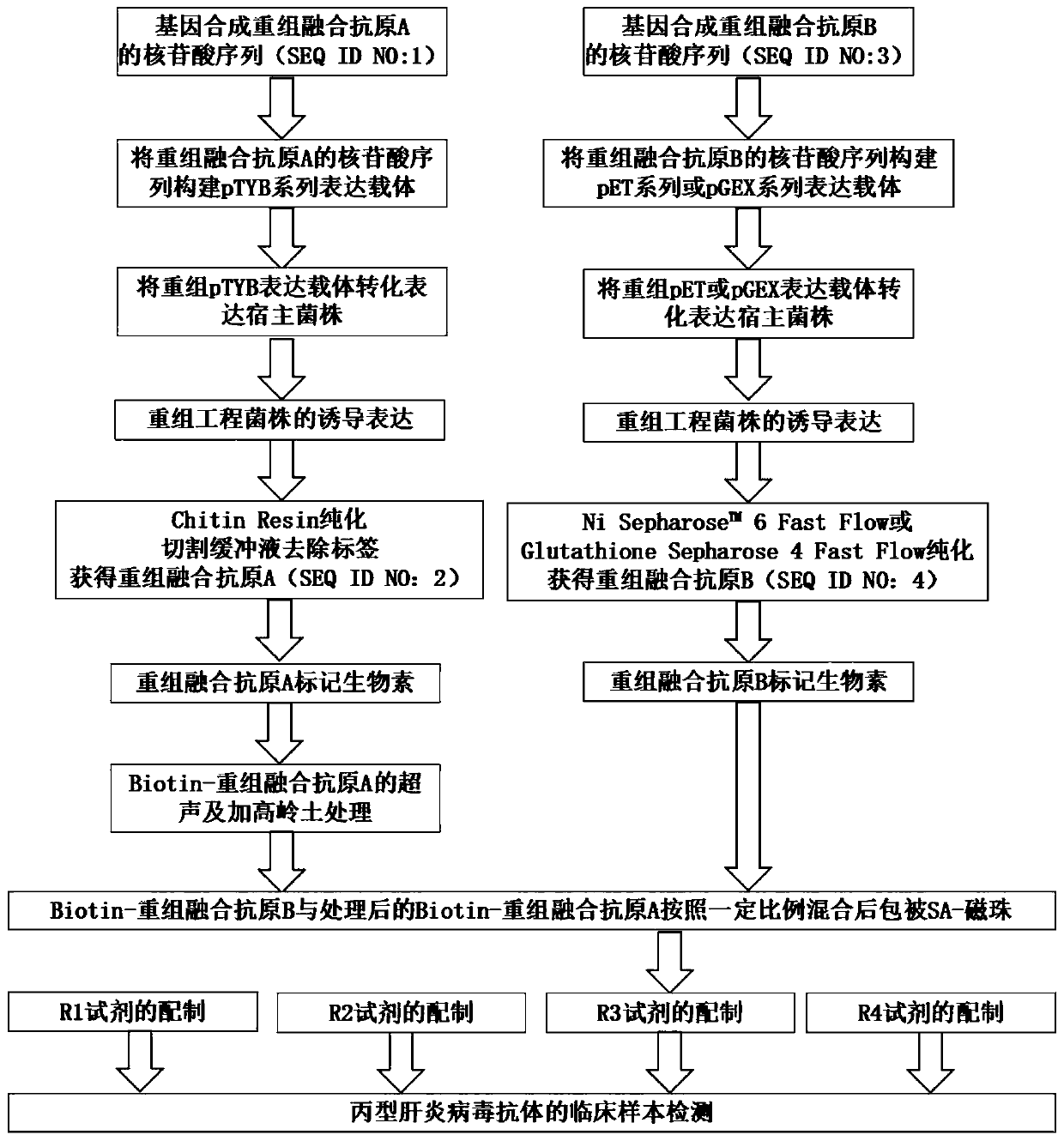
[0038] Example 1: Preparation of recombinant fusion antigen A:
[0039] What this embodiment discloses is the preparation method of recombinant fusion antigen A, specifically as follows:
[0040] 1. Artificially synthesized recombinant fusion antigen A gene sequence and constructed expression vector:
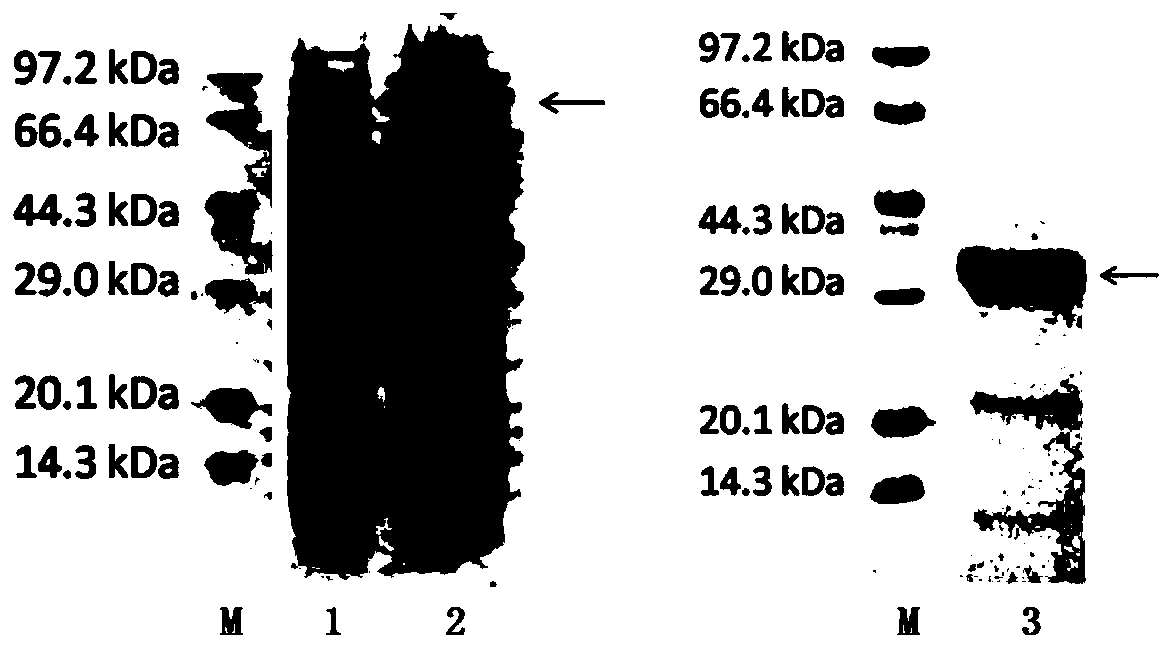
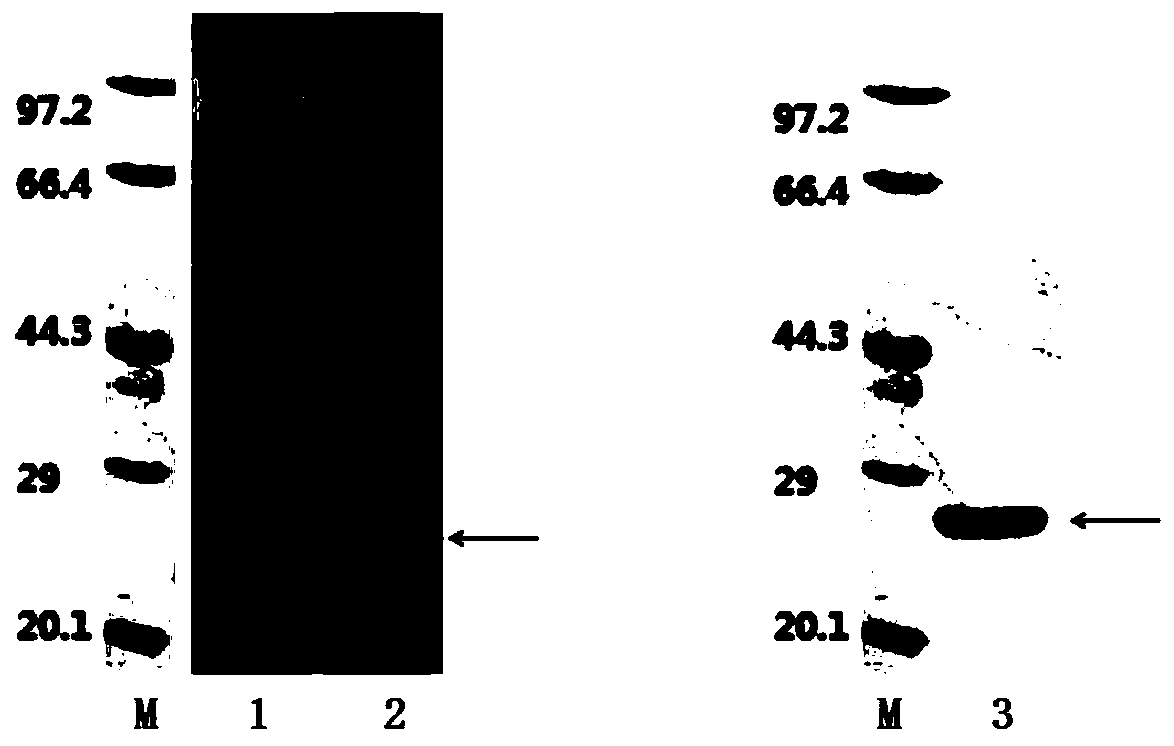
[0041] Sangon Bioengineering (Shanghai) Co., Ltd. artificially synthesized the gene according to the sequence information of the recombinant fusion antigen A gene, and its sequence is SEQ ID NO:1. The gene is linked with the pMD-18 vector after gene synthesis is completed, and the 5' end of the recombinant fusion antigen A gene sequence is designed with a BamH I restriction site, and the 3' end is designed with a terminator and an EcoR I restriction site. The recombinant fusion antigen A gene sequence was double-digested from the pMD-18 vector with BamH I and EcoR I restriction sites, and the pTYB21 vector expression vector was double-digested with BamH I and EcoR I restricti...
Embodiment 2
[0052] Example 2: Preparation of recombinant fusion antigen B:
[0053] This embodiment discloses a preparation method of recombinant fusion antigen B, specifically as follows:
[0054] 1. Artificially synthesized recombinant fusion antigen B gene sequence and constructed expression vector:
[0055] Sangon Bioengineering (Shanghai) Co., Ltd. artificially synthesized the gene according to the sequence information of the recombinant fusion antigen B gene, and its sequence is SEQ ID NO:3. After the gene is synthesized, it is linked to the pMD-18 vector. The 5' end of the recombinant fusion antigen B gene sequence is designed with an EcoR I restriction site, and the 3' end is designed with a terminator and an XhoI restriction site. Use the combination of EcoR I and XhoI restriction sites to double-digest the recombinant fusion antigen B gene sequence from the pMD-18 vector, and use the combination of EcoR I and XhoI restriction sites to double-digest the pET28a(+) expression ve...
Embodiment 3
[0066] Example 3: GST-recombinant fusion antigen B:
[0067] The difference between this example and Example 2 is that this example discloses a GST-recombinant fusion antigen B, which has a GST tag, and the specific preparation method differs from Example 2 only in that:
[0068] The expression vector in step (1) is pGEX-4T-1; the control in step (2) adopts the host bacterium transformed into pGEX-4T-1 empty vector; in step (3), add GST Loadingbuffer to replace His Loading buffer, GST ElutionBuffer to replace His Elution Buffer, Glutathione Sepharose 4Fast Flow affinity column replaces NiSepharose TM 6Fast Flow affinity column, PBS buffer replaces Tris buffer.
[0069]Among them, the composition of GST Loading buffer is: NaCl 8.18g / L, KCl 0.2g / L, NaCl 2 HPO 4 3.58g / L, KH 2 PO 4 0.25g / L, pH7.3. The composition of GST Elution Buffer is: Tris 6.06g / L, reduced glutathione 3.04g / L, pH8.0. The composition of PBS buffer is: Na 2 HPO 4 5.8g / L, NaH 2 PO 4 0.6g / L, NaCl 9.0g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com