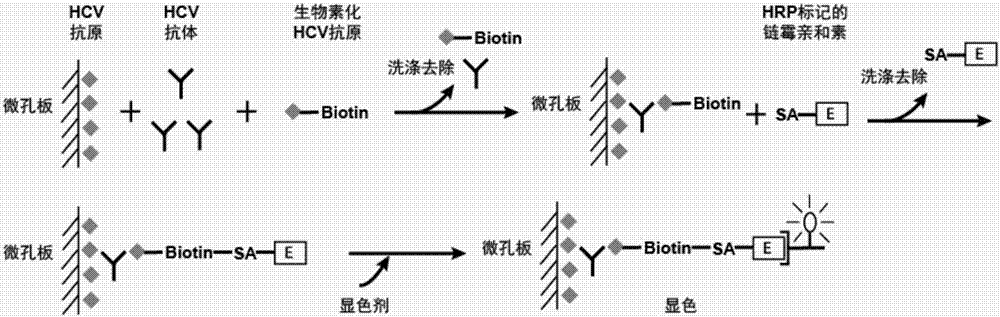
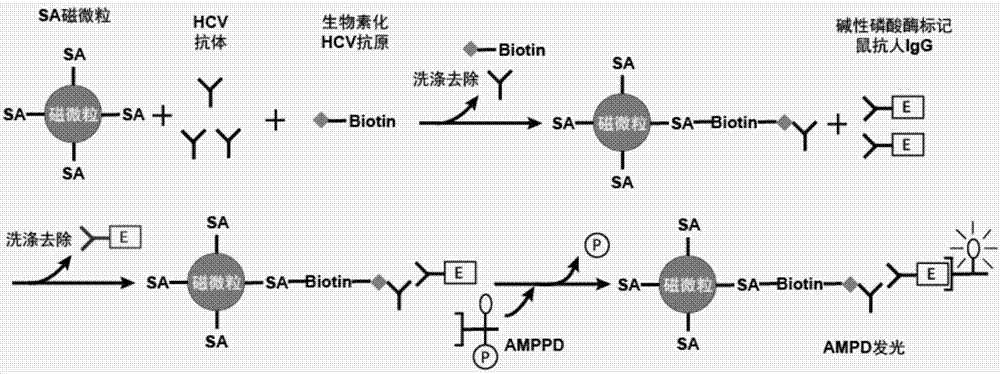
Hepatitis C antigen pretreatment method, and detection kit
A detection kit and pretreatment agent technology, applied in the field of in vitro diagnostic medical testing, can solve problems such as unfavorable commercialization, difficult detection, and difficult control of the processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Correspondingly, the present invention also provides a method for preparing the biotinylated hepatitis C antigen pretreated above, the method comprising the following steps:
[0054] 1) Add a pretreatment agent to the hepatitis C antigen and react for a period of time;
[0055] 2) using biotin to cross-link the treated hepatitis C antigen;
[0056] 3) Preserving the cross-linked biotinylated hepatitis C antigen in a diluent containing a pretreatment agent.
[0057] In a specific embodiment, the following process parameters are used in the preparation process of the biotinylated pretreated hepatitis C antigen of the present invention with excellent detection sensitivity and stability of hepatitis C virus antibody.
[0058] In a specific embodiment, the hepatitis C antigen pretreatment agent is 4-nitrothiophenol or sodium 2-mercaptoethanesulfonate; preferably sodium 2-mercaptoethanesulfonate.
[0059] In another preferred embodiment, the hepatitis C antigen pretreatment...
Embodiment 1
[0100] Example 1. Preparation of biotinylated pretreated hepatitis C antigen
[0101] 1) Dilute hepatitis C antigen and sodium 2-mercaptoethanesulfonate to 1 mg / mL and 1% (w / v) with pH 7.4, 20 mM PBS, respectively;
[0102] 2) Add sodium 2-mercaptoethanesulfonate solution to the hepatitis C antigen at a ratio of 1:10, mix well, place at 2-8°C, and react for 30 minutes;
[0103] 3) Dilute the pretreated hepatitis C antigen to 1mg / mL with pH7.4, 20mM PBS;
[0104] 4) Dissolving Biotin containing a sulfhydryl functional group in purified water to prepare a 2 mg / mL Biotin solution;
[0105] 5) According to the ratio of 1:15, add Biotin solution containing sulfhydryl functional group to the pretreated hepatitis C antigen;
[0106] 6) Mix well, place at room temperature, and react for 30 minutes;
[0107] 7) Dialyze overnight in 20mM PBS solution at pH 7.4;
[0108] 8) Add glycerol at a volume ratio of 1:1, mix well, and store at -20°C to prepare biotinylated pretreated hepatiti...
Embodiment 2
[0109] Example 2. Preparation of Hepatitis C Virus Antibody ELISA Detection Kit
[0110] 1. Preparation of Biotinylated HCV Antigen Reagent
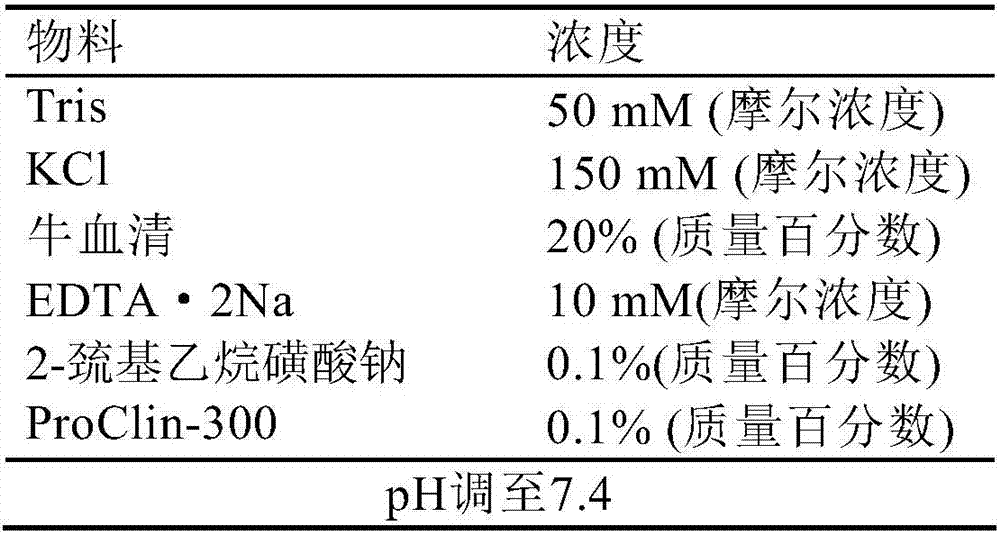
[0111] Biotinylated Hepatitis C Antigen Reagent Diluent Formula:
[0112]
[0113] Dilute the biotinylated hepatitis C antigen with diluent to 0.2 μg / mL, and mix thoroughly at room temperature for at least 30 minutes to prepare the biotinylated hepatitis C antigen reagent.
[0114] 2. Preparation of Streptavidin Enzyme Reagent
[0115] Streptavidin Enzyme Reagent Diluent Recipe:
[0116]
[0117] Dilute horseradish peroxidase-labeled streptavidin with enzyme diluent to 1:1000-1:5000, and mix thoroughly at room temperature for at least 30 minutes to prepare streptavidin enzyme reagent.
[0118] 3. Preparation of Washing Solution
[0119] Washing liquid formula:
[0120]
[0121]
[0122] Prepare the washing solution according to the formula, and mix thoroughly at room temperature for at least 30 minutes to obtain the wash...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com