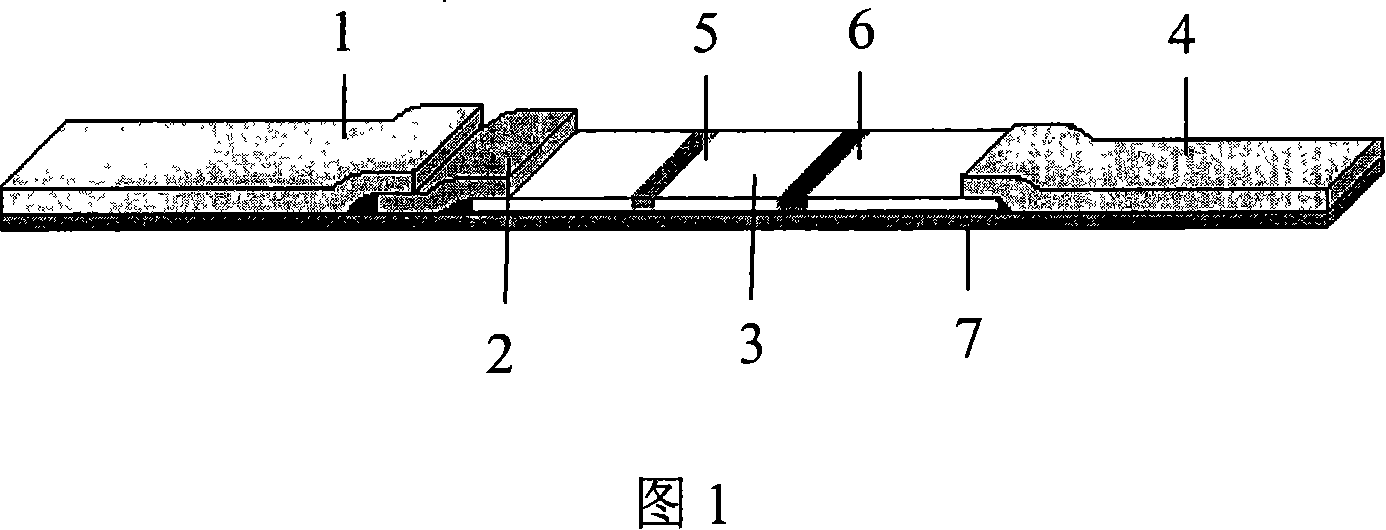
Hepatitis C virus antibody quick diagnosis test paper and its preparation method
A hepatitis C virus, rapid diagnosis technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of improving sensitivity, strong specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of HCV antibody rapid diagnostic test paper
[0023] 1 main material
[0024] 1.1 Mixed recombinant antigen: CORE of HCV, NS3 fusion antigen: products of Biotech Atlantic Inc (BBI) and the Academy of Military Medical Sciences of the United States, respectively used for coating and labeling of test paper; anti-HCV antibody: product of BBI company; chloroauric acid: Sigma Company products, nitrocellulose (NC) membrane: products of Millipore; bovine serum albumin (BSA), polyethylene glycol (PEG) 20000, hydrolyzed casein: products of Sigma. Other commonly used reagents are analytical reagents.
[0025] 1.2 National reference product of HCV antibody (colloidal gold): developed by China Institute for the Control of Pharmaceutical and Biological Products. Including 20 positive sera, numbered P1-P20, 20 negative sera, numbered N1-N20, 4 sensitivity sera, numbered L1_1, L1_2, L2_1, L2_2, 1 precision serum, numbered J.
[0026] 2 methods
[0027]...
Embodiment 2
[0034] Embodiment 2: Performance analysis of HCV antibody rapid diagnostic test paper
[0035] 1 main material
Embodiment 1
[0038] See embodiment one for specific instructions;
[0039] 1.3 Serum containing interfering substances: including 50 serums of common interfering substances hyperlipidemia, hemolysis, and jaundice, 50 serums each containing positive antibodies to related infectious diseases HAV, HBV, HIV, TP, and HP, and containing different anticoagulants heparin, EDTA, and 20 copies of sodium citrate plasma were collected, verified and preserved by our company in relevant hospitals in Tianjin. The above serum or plasma were tested by more than two ELISAs and all were HCV antibody-negative serum.
[0040] 1.4 The clinical serum was collected by the company in relevant hospitals in Tianjin, a total of 1180 samples were collected for outpatient testing.
[0041] 1.5 HCV antibody diagnostic kit (ELISA): Shanghai Kehua Bioengineering Co., Ltd., Beijing Wantai Bio-Pharmaceutical Co., Ltd. and Pusheng (Tianjin) Technology Co., Ltd.
[0042] 2 methods
[0043] 2.1 Sample detection method: see E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com