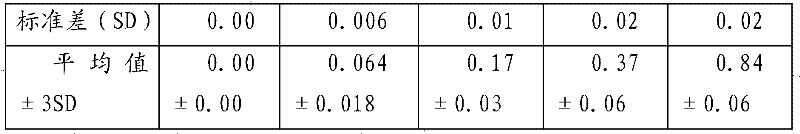Theophylline homogeneous enzyme immunoassay kit and preparation method thereof
A detection kit and homogeneous enzyme immunoassay technology, applied in the field of medical testing, can solve the problems of high price, short validity period, cumbersome operation, etc., and achieve the effects of good stability and repeatability, rapid detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Synthesis of Theophylline Immunogen
[0037] a. Dissolve 200 mg of bovine serum albumin (BSA) in 50 mL of 0.2 mol / L, pH 8.5 phosphate buffer;
[0038] b. Add the following components into a beaker and stir to dissolve: 100mg theophylline derivatives, 3.5mL dimethylamide (DMF), 3.5mL ethanol, 7.0mL (10mmol / L, pH5.0) potassium phosphate buffer, 400mg1 -Ethyl-3-(-3-dimethylaminopropyl)carbodiimide, 50mg of N-hydroxysulfosuccinimide, stirred and dissolved for 30min;
[0039] Add the solution dissolved in b above dropwise to the BSA solution in a above, and stir overnight at 2-8°C to obtain the antigen;
[0040] The synthesized antigen is purified by dialysis to obtain theophylline immunogen.
[0041] 2. Preparation of anti-theophylline-specific antibody
[0042] Dilute the synthetic theophylline immunogen to 1.0 mg / mL with PBS phosphate buffer solution, then mix 1.0 mL of antigen solution with Freund's complete adjuvant, and inject it into rabbits; 14-21 days later, u...
Embodiment 2
[0054] Embodiment 2 utilizes automatic biochemical analyzer to carry out sample test
[0055] (1) Collection of serum samples, collect serum samples according to conventional methods;
[0056] (2) According to the operating instructions of the Hitachi 7180 automatic biochemical analyzer, turn on the instrument, perform the optical density detection of the instrument and clean the probe, and check whether the instrument is operating normally;
[0057] (3) After the instrument is running normally, put the reagents R1 and R2 into the reagent compartments of R1 and R2 in turn, and put the serum sample into the sample tray 1 (S1), 0.0μg / mL, 2.5μg / mL, 5.0μg / mL, 10.0μg / mL Put the theophylline calibration solution of μg / mL, 20.0μg / mL, and 40.0μg / mL into the designated position of the sample plate 2 (S2);
[0058] (4) When the instrument is in the Stand by state, set the operation procedure and inspection parameters of theophylline, and the specific inspection parameters are shown in ...
Embodiment 3
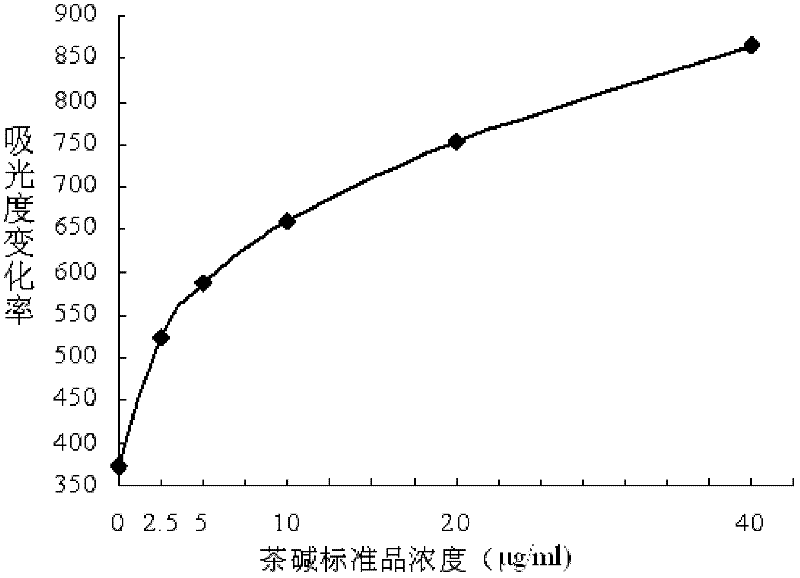
[0064] 1. Preparation of theophylline homogeneous enzyme immunoassay calibration curve
[0065] see figure 1 , the homogeneous enzyme immunoassay of theophylline was carried out with an automatic biochemical analyzer, and the reaction system could be further optimized by adjusting the ratio of reagent R1 and reagent R2. mL, 5.0 μg / mL, 10.0 μg / mL, 20.0 μg / mL, 40.0 μg / mL. The working volume of the calibration solution is 10-35 μL, then add 100-200 μL reagent R1 and 100-200 μL reagent R2, use the two-point rate method to detect the change rate of absorbance at the main wavelength of 340 nm and the secondary wavelength of 405 nm, establish and optimize the tea Alkaline homogeneous enzyme immunoassay calibration curve, the establishment and optimization of the calibration curve was completed on Hitachi 7180 automatic biochemical analyzer. Get a working standard curve as figure 1 shown.
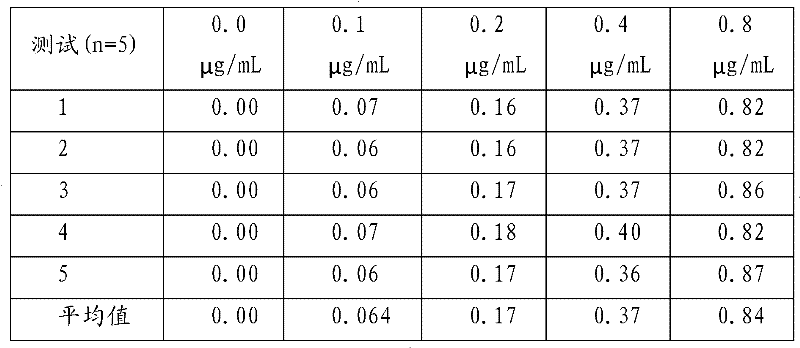
[0066] 2. Sensitivity test
[0067] Add unequal amount of theophylline standard substance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com