Procalcitonin light-initiated chemiluminescence immunoassay kit and preparation method thereof
A photo-excited chemiluminescence and procalcitonin technology, applied in the field of procalcitonin photo-excited chemiluminescence immunoassay kits, can solve the problems of EIA sensitivity limitation, easy inactivation of enzyme activity, radionuclide contamination, etc. Wide detection range, high sensitivity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation kit
[0028] Preparation of receptor microspheres coated with anti-procalcitonin monoclonal antibody: add 0.2 mg of PCT monoclonal antibody to a centrifuge tube with a filter membrane, centrifuge at 8 000 r / min for 5-6 min, and use labeling buffer After repeated washing 6 times with the antibody solution (0.13mol / L, pH 8.0PBS), 1 mg of receptor microspheres, 10 μL of 25 mg / mL NaBH3CN (prepared with labeling buffer), 1.25 μL of 10% Tween-20 were added to the antibody solution. The volume of labeling buffer was supplemented to 200 μL, and the reaction was shaken at 37°C for 48 hours in the dark. Add 10 μL of 65 mg / mL L CMO (prepared with 0.8M NaOH) to block unbound sites, incubate at 37°C for 1 hour in the dark, and then centrifuge and wash to obtain antibody-linked receptor microspheres, which are diluted for later use.
[0029] PCT calibrator: use standard buffer (50mmol / L Tris-HCl, 1.5%BSA, 0.9%NaCl, 0.05%Proclin-300, 0.01%Tween-20, pH7.8) to pre...
Embodiment 2
[0041] Example 2 Evaluation test
[0042] Reagents: PCT calibrators, receptor microspheres coated with anti-procalcitonin monoclonal antibody, biotinylated anti-procalcitonin monoclonal antibody, streptavidin- donor microspheres.
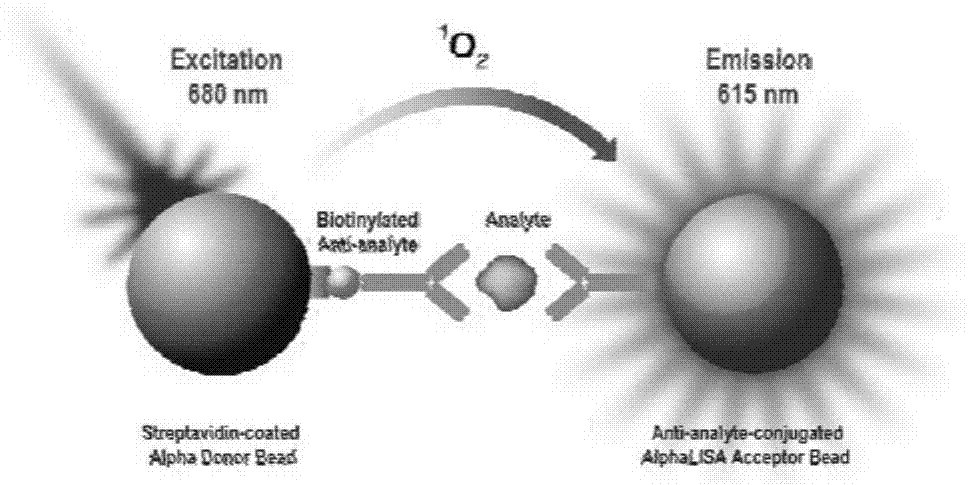
[0043] Detection method: add calibrator, receptor microspheres coated with anti-procalcitonin monoclonal antibody, and biotinylated anti-procalcitonin monoclonal antibody into the wells of white opaque plates, incubate at 37°C with shaking 15min, then add 175μL of streptavidin-based donor microspheres, incubate at 37°C with shaking for 15min, detect the signal value on the AlphaScreen / Lisa detector, and calculate the PCT content of the tested sample from the standard curve, the unit is ng / ml, at the same time judge the negative and positive of the sample according to the S / CO value, and finally print the test report.
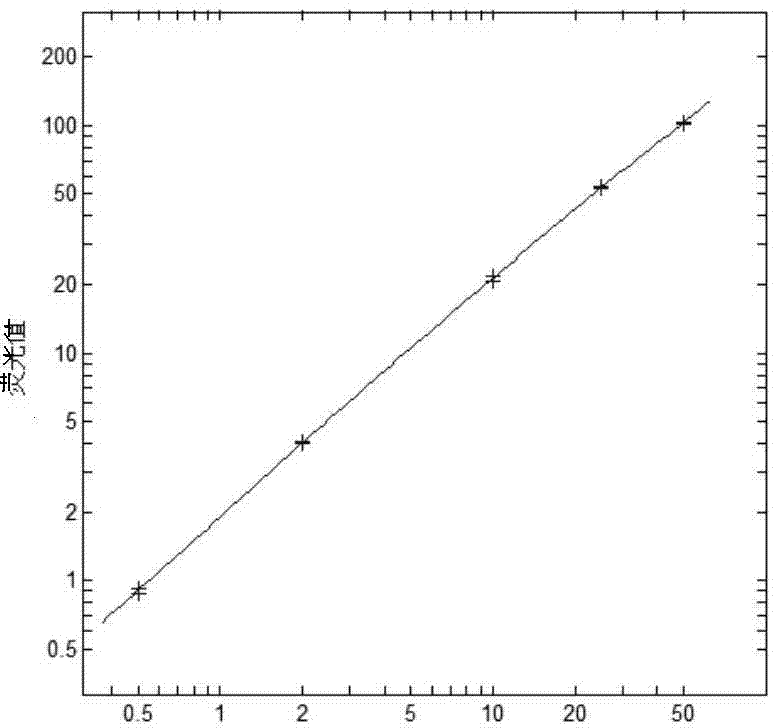
[0044] 1. Detection of linear range
[0045] The blood samples of 405 normal people were tested at 3 clinical trial sites, and 9...
Embodiment 3
[0067] Example 3 Clinical comparative test
[0068] Reagents: PCT calibrators, receptor microspheres coated with anti-procalcitonin monoclonal antibody, biotinylated anti-procalcitonin monoclonal antibody, streptavidin- donor microspheres.
[0069] Sample source: 126 clinical serum samples were from systemic bacterial, fungal and parasitic infections, systemic inflammatory response syndrome, sepsis, acute and chronic pneumonia, acute pancreatitis, active hepatitis, trauma, etc. 405 were healthy individuals.
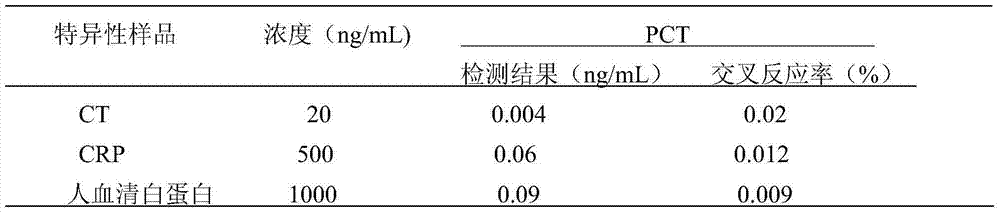
[0070] Serum samples were tested by PCT-TRFIA and the chemiluminescence immunoassay kit (ILMA) of BRAHMS company abroad. The correlation analysis was carried out by SPSS17.0 software. There is a significant correlation between the detection results of , and the correlation equation is Y AlphaLisa =1.155X ILMA -0.354; Comparing the detection performance of the two methods, it can be seen that there is no significant difference between the photo-excited chemiluminescence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com