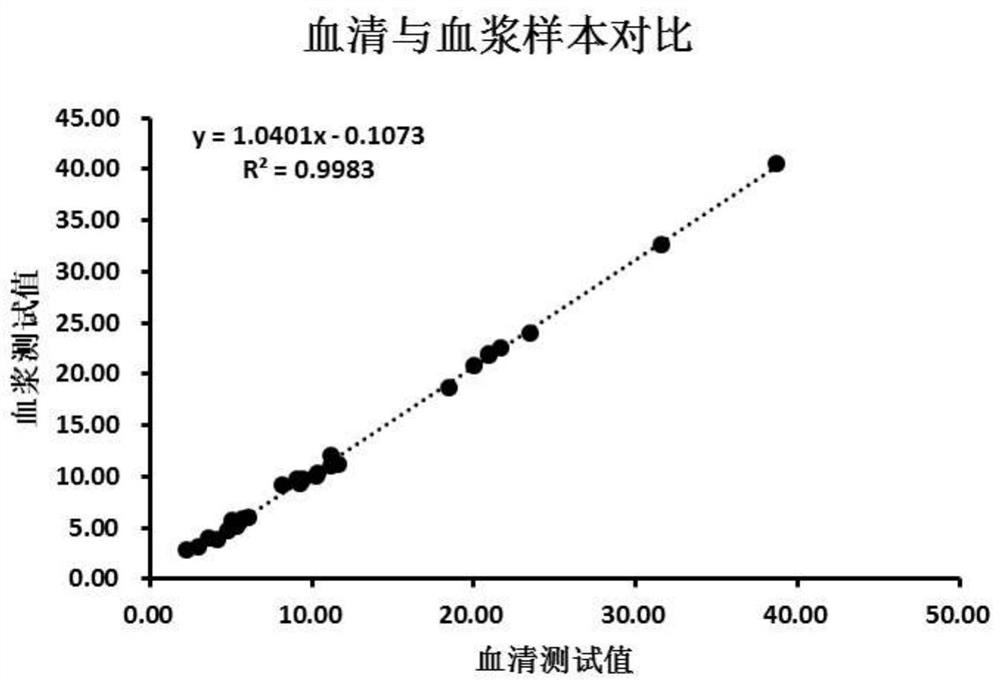
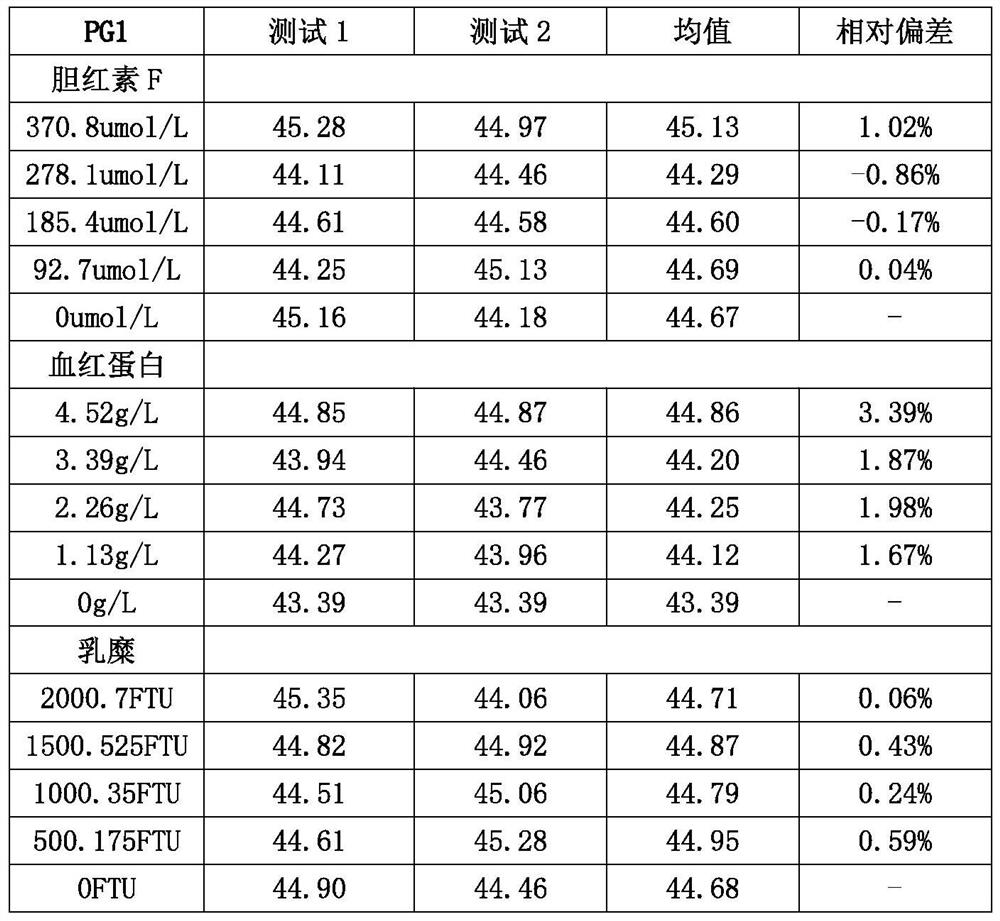
Pepsinogen II monoclonal antibody and application thereof
A monoclonal antibody, antigen-antibody technology, applied in immunoglobulins, anti-enzyme immunoglobulins, instruments, etc., can solve the problems of low antibody activity, poor affinity, and susceptibility to lipoprotein interference, avoiding interference and low requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Expression and purification of recombinant PGII
[0043] The human PGII gene sequence was cloned into a prokaryotic expression vector to construct a prokaryotic expression plasmid, and the expression plasmid was transformed into Escherichia coli BL21 and cultured on an LB plate containing antibiotics. Single clones were picked and inoculated into LB medium. After culturing until the OD value of the bacterial solution reached 0.6, IPTG was added to induce protein expression. After 16 hours of induction, the bacterial liquid was collected by centrifugation. The supernatant was collected by centrifugation after the cells were crushed, and purified by ion exchange and molecular sieve to obtain the antigenic protein.
Embodiment 2
[0044] Example 2: Mouse immunization and antibody detection
[0045] Five female BALB / c mice of SPF grade aged 6-8 weeks were selected, and Freund's complete adjuvant and PGII protein at a concentration of 2 mg / ml were mixed and emulsified in equal volumes. The emulsified antigen was immunized with 6-8 week-old SPF grade female BALB / c mice, and each mouse was injected with 40 μg of antigen protein by sole injection or back subcutaneous injection. Two weeks after the completion of the primary immunization, the antigenic protein was mixed with Freund's
[0046] The incomplete adjuvant was mixed and emulsified, and each mouse was injected with 40 μg of antigenic protein by plantar injection or back subcutaneous injection. Two weeks later, blood was collected through the tail vein, the supernatant was collected by centrifugation and the serum titer was detected by ELISA. Immunize once every two weeks and test the serum titer. After the second immunization, the serum titer after...
Embodiment 3
[0047] Example 3: Production and purification of monoclonal antibodies
[0048] Two groups of 6-8 week BALB / c mice were selected, and 500 μL paraffin oil was injected intraperitoneally to suppress the immune response of the mice. One week after the injection, 0.5 ml of PGII hybridoma cells were intraperitoneally injected into a group of mice, and the number of cells was about 1×10 6 quantity. Ascites collection began two weeks later. The collected ascites was subjected to ammonium sulfate precipitation and protein A affinity purification to obtain the target antibody.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com