Soluble secretory expression of PG II-MBP fusion protein and application thereof
A fusion protein, MBP-PGII technology, applied in the field of soluble secretory expression and preparation of recombinant PG II fusion protein, can solve the problems of difficult industrialization, limited sources, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Amplification of PG II coding sequence
[0015] Use the PCR method to amplify the coding sequence of PG II from the plasmid PET32a-PG II (constructed by our company). The 5' end of the primer PGC5 used has added the restriction site of BamH I, and the 5' end of the primer PGC3 has added HindIII enzyme cleavage site.
[0016] PGC5: 5'-aagggatccggtagcggctctggctcgggttctggcgcagttgtcaaggttcctttgaag-3'
[0017] PGC3: 5'-aagaagcttttagtggtggtggtggtggtggtggtgagcagcggtagcaaatccgac-3'
[0018] 1 μl of La Taq DNA polymerase (TaKaRa), 4 μl of 10 μM PGC5 and 4 μl of PGC3, 8 μl of 2.5 mM dNTP, and 10 μl of 10×PCR reaction buffer were added to 100 μl of the PCR reaction system. The reaction conditions of PCR were: pre-denaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 57°C for 30 seconds, extension at 72°C for 1 minute and 30 seconds, and then back to pre-denaturation for a total of 25 cycles, with a final extension of 7 minutes. The tar...
Embodiment 2
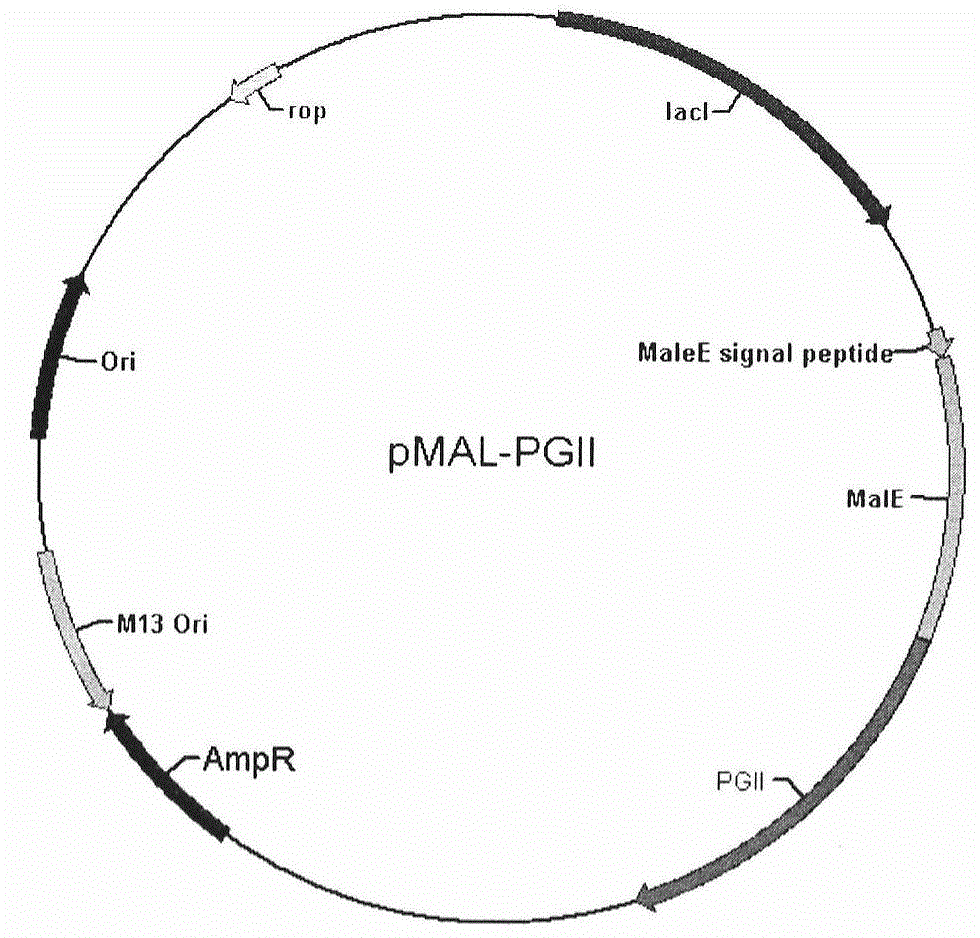
[0019] Embodiment 2: Construction of MBP-PG II fusion protein expression vector
[0020] Take 2 μg of the PG II coding sequence obtained by PCR amplification in Example 1, and establish a 50 μl double enzyme digestion system, specifically as follows: 2 μg of the PG II coding sequence, 5 μl of 10×K enzyme digestion reaction buffer, 1.5 μl of BamH I and Add 1.5 μl of Hind III to a total volume of 50 μl with double distilled water, and react in a water bath at 37°C for 6 hours. The double digestion product of the PG II coding sequence was purified using a gel extraction kit (OMEGA BIO-TEK).
[0021] Take 600ng of pMAL-p2X (NEB Company) vector, and set up 50 μl of double enzyme digestion system, as follows: 600ng of pMAL-p2X vector, 5 μl of 10×K digestion reaction buffer, 1.5 μl of BamH I and 1.5 μl of HindIII, add double-distilled water to a total volume of 50 μl, and react in a 37°C water bath for 6 hours. The pMAL-p2X vector double digestion product was purified using a gel e...
Embodiment 3
[0024] Embodiment 3: Expression of MBP-PG II fusion protein
[0025] The obtained pMAL-PG II plasmid was transformed into E.coli BL21(DE3) (Novagen Company) competent cells. Induced expression of bacteria: the clones were picked and inoculated in 100 ml LB liquid medium containing 100 μg / ml ampicillin, cultured overnight at 37° C. and 250 rpm to prepare seed solution.
[0026] Take 10ml of seed solution and inoculate into a 3L Erlenmeyer flask filled with 1L LB-Amp medium, and culture at 37°C until OD 600 0.5, then add IPTG with a final concentration of 0.5mM, lower the temperature to 28°C for 8 hours, and then centrifuge at 12000g to obtain the bacteria. Whole bacteria electrophoresis samples were prepared, and protein electrophoresis was used to analyze the expression of the target protein. The sample preparation method, electrophoresis voltage, gel staining and decolorization methods for SDS-PAGE electrophoresis are described in the second edition of "Molecular Cloning Ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com