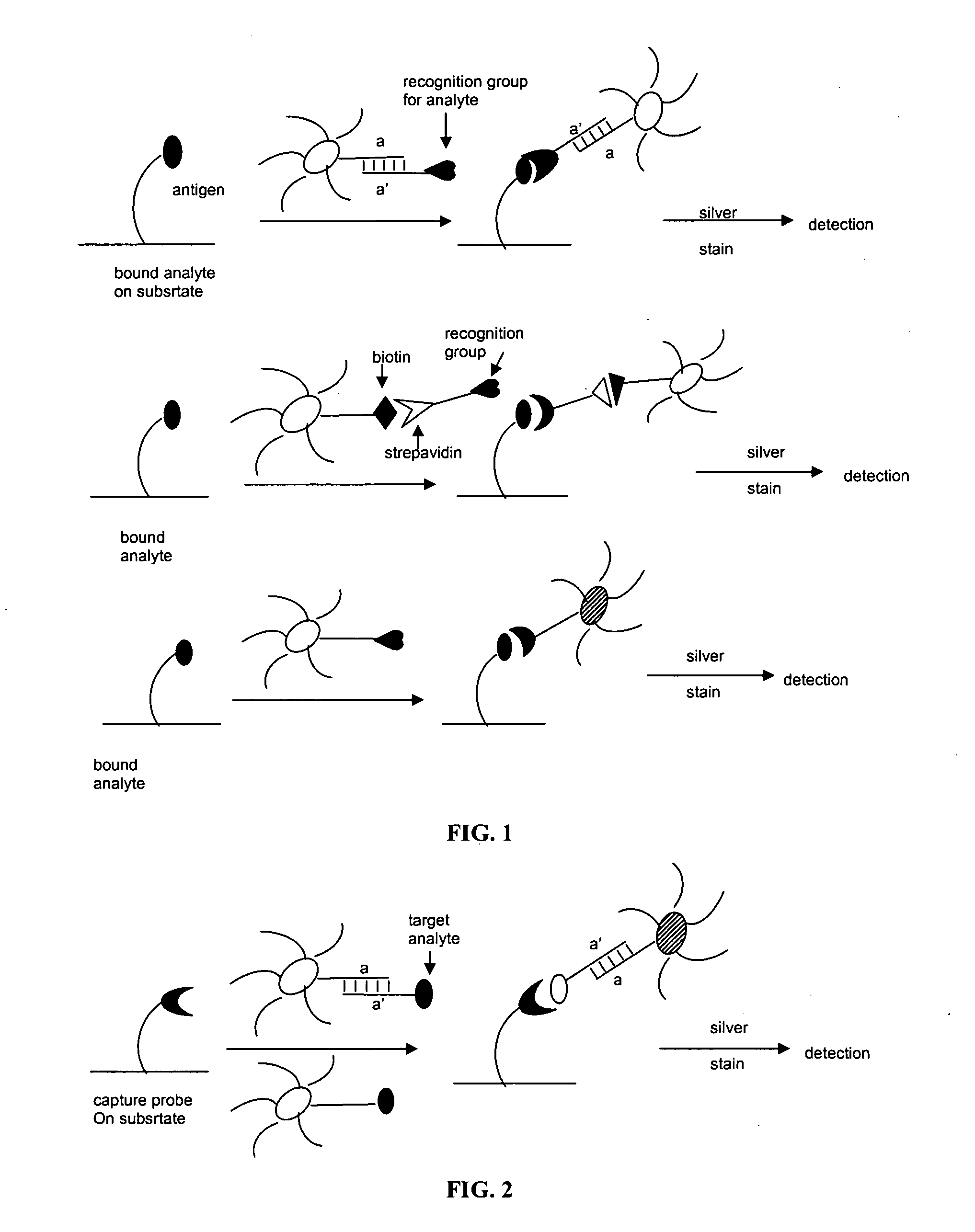
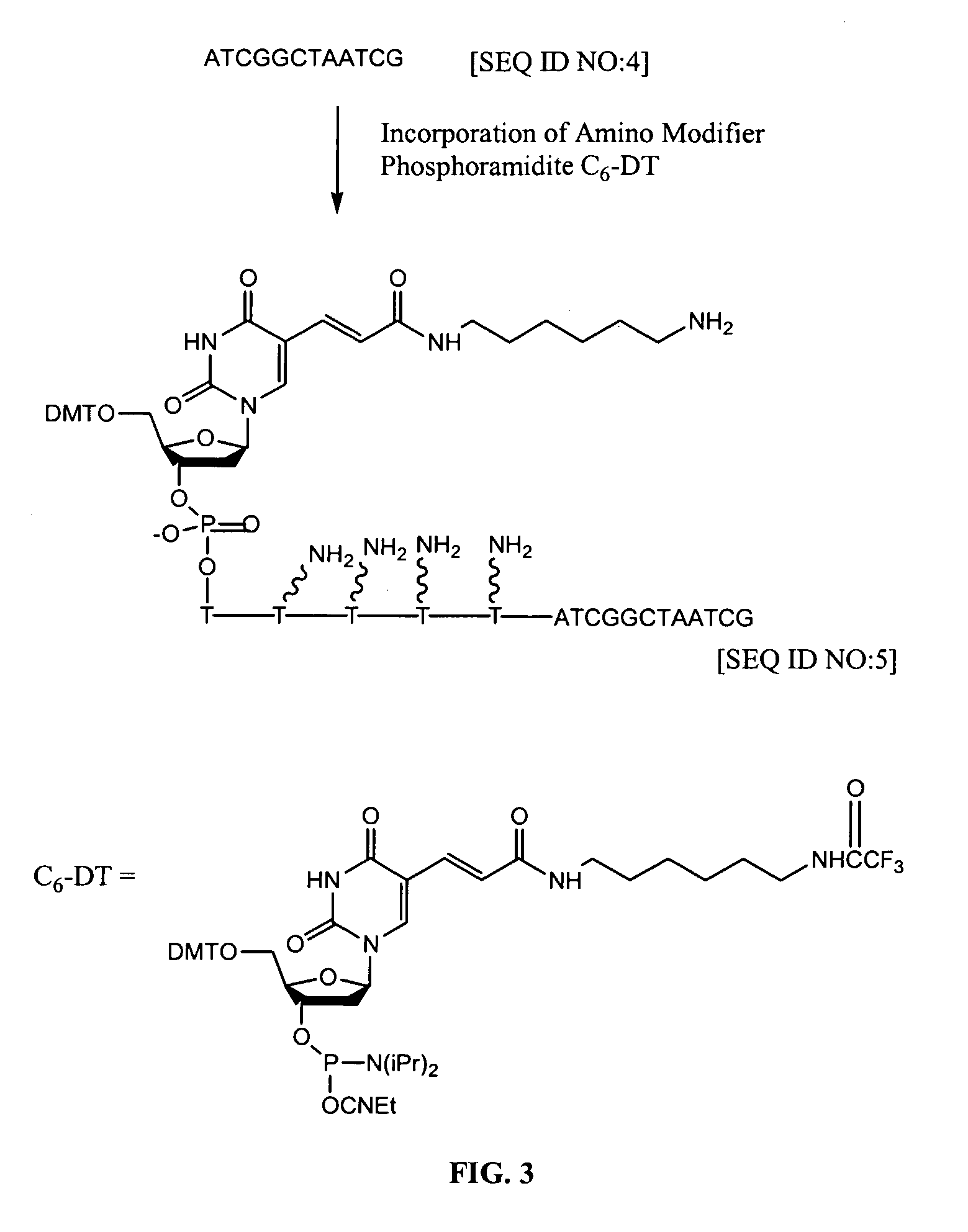
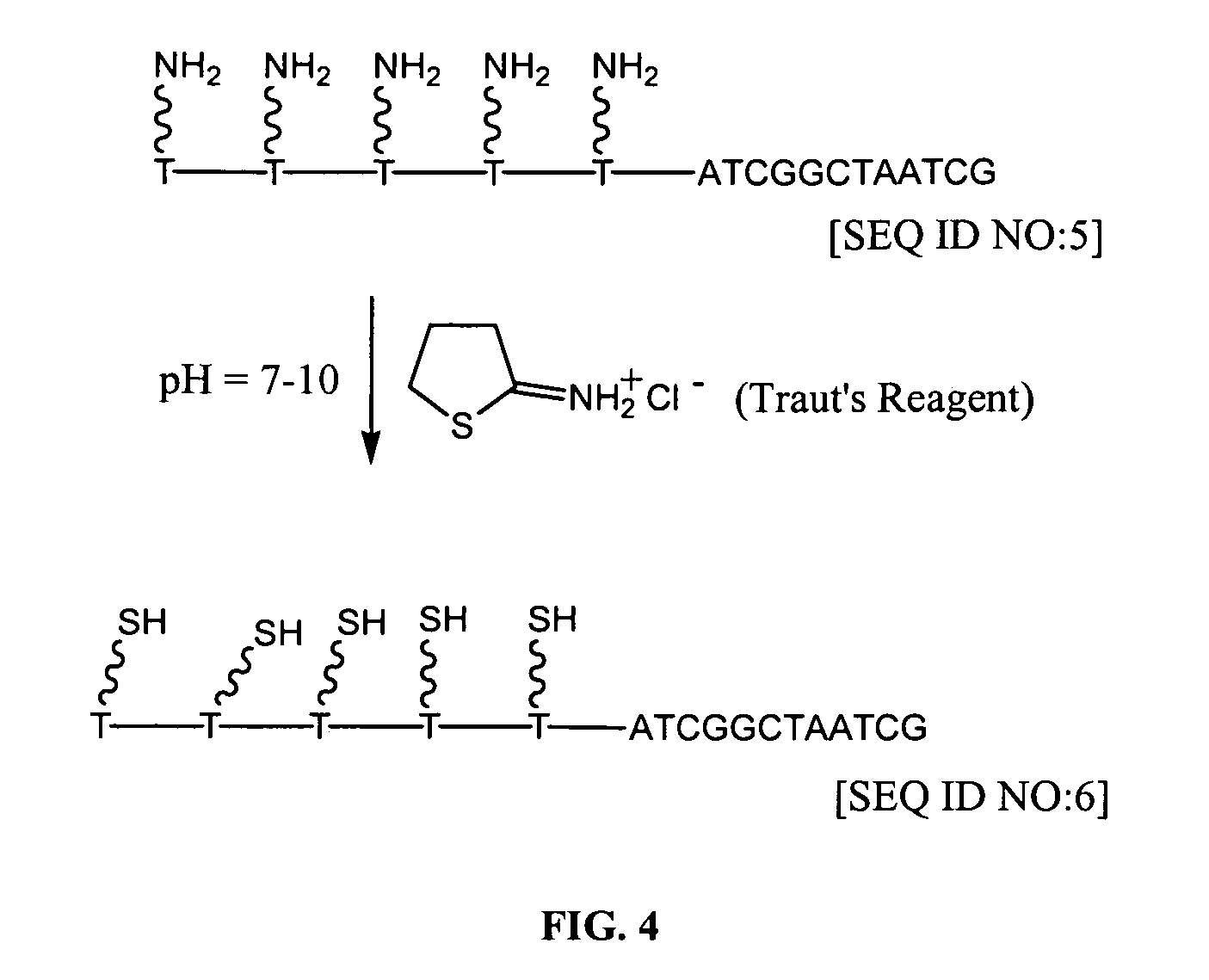
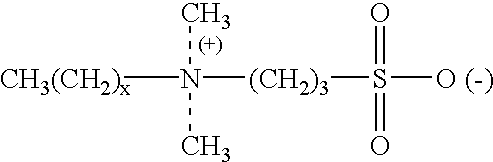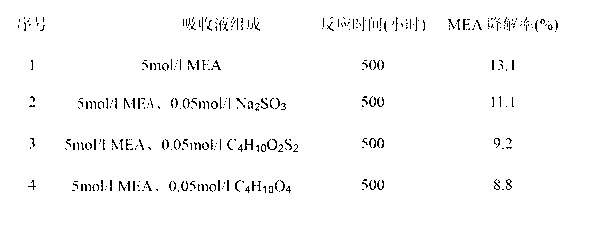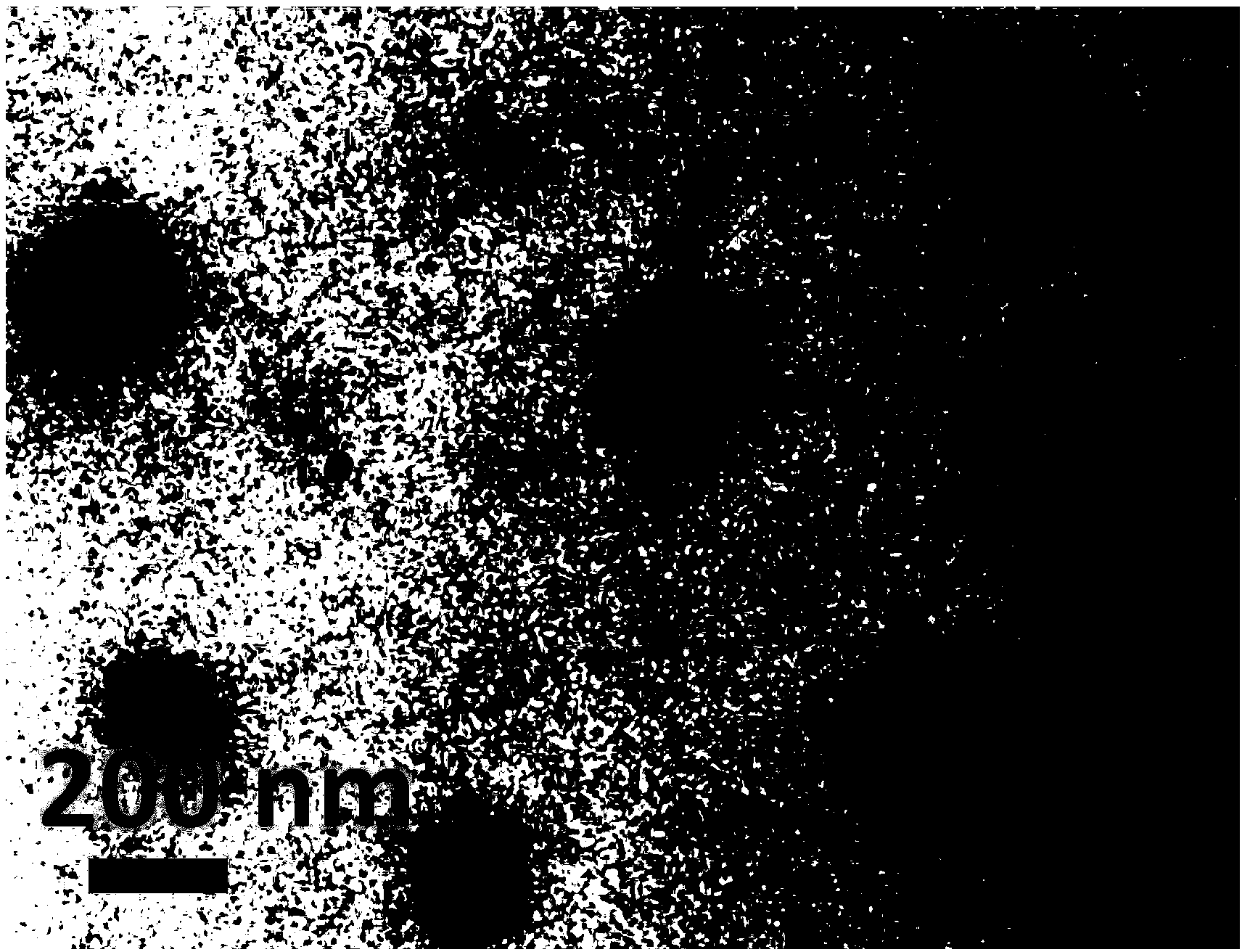Patents
Literature
336 results about "Clelands Reagent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
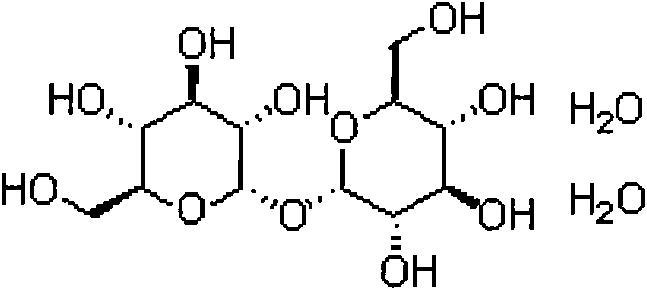
Dithiothreitol (DTT) is the common name for a small-molecule redox reagent also known as Cleland's reagent. DTT's formula is C4H10O2S2 and the chemical structure of one of its enantiomers in its reduced form is shown on the right; its oxidized form is a disulfide bonded 6-membered ring (shown below).
A liquid-based cell preservation solution
The invention relates to a pathological examination, in particular to a liquid-based cell preservation solution used in cytopathological examination of human cervical mucus, sputum, urine, pleural fluid, tracheal mucus and the like. It is characterized in that it is prepared from alcohols, sodium phosphate buffer, edetate disodium, sodium chloride 0.08%-0.12%, potassium chloride, formaldehyde, dithiothreitol, calcium acetate, magnesium acetate, etc. As a result, it can not only maintain the stability of the cell structure. It can reduce the agglomeration and precipitation of cell mucus and the loss of cell rupture, and can also make cells easy to stain, improve the clarity of cell preparation, facilitate the smooth progress of pathological examination, and the cost is low, which is conducive to popularization and use.
Owner:XIAOGAN CENT HOSPITAL +1
Bioconjugate-nanoparticle probes
The invention provides nanoparticle-bioconjugate probes that are useful for detecting target analytes such as nucleic acids. The probes of the invention are stable towards heat and resistant to displacement by thiol containing compounds such as DTT (dithiothreitol).
Owner:NANOSPHERE INC
Hair analysis method
InactiveUS6949344B1Reliable solubilizationAccurate methodPre-tanning chemical treatmentMicrobiological testing/measurementBetaineDigestion
A method the direct analysis of an analyte in keratinized structures, e.g., hair and fingernails, which comprises preparing a mixture containing a low redox potential activator compound such as dithiothreitol or dithioerythritol, an enzyme suitable for the digestion of the keratin structure, a sample of the keratin structure and a biological detergent that aids the digestion of the keratinized structure at a relatively low pH, e.g., between about 6.2 and 8; permitting the enzyme to at least substantially digest the sample of keratin structure, and subjecting the digest solution to analysis, preferably by radioimmunoassay, to determine the identity and amount of analyte in the keratin structure sample. To accelerate the method, cupric sulfate may be added to the mixture after degradation of the keratin sample. The enzyme may be a peptidase, endopeptidase or proteinase, with papain, chymopapain, and proteinase K being preferred for use in the invention. The preferred biological detergents include betaine, sulfo-betaine, alkylglucosides and bile acids.
Owner:PSYCHEMEDICS CORPORATION
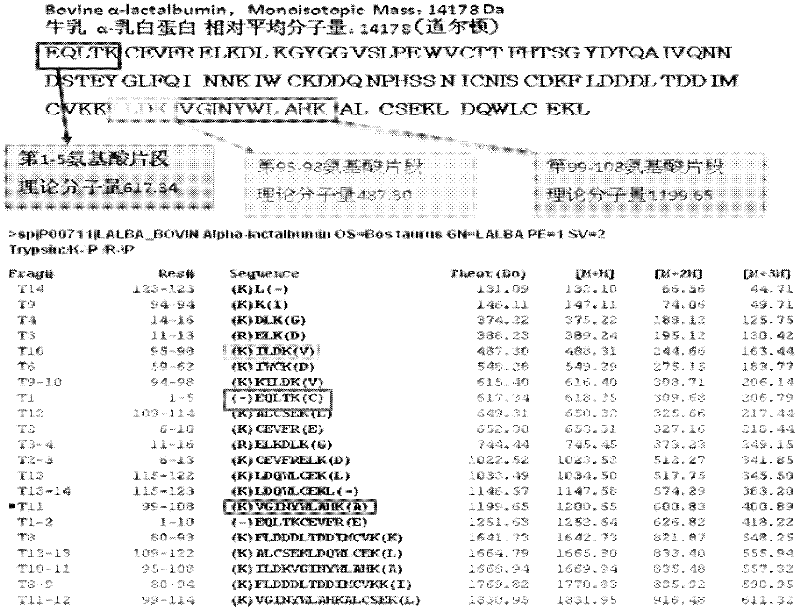
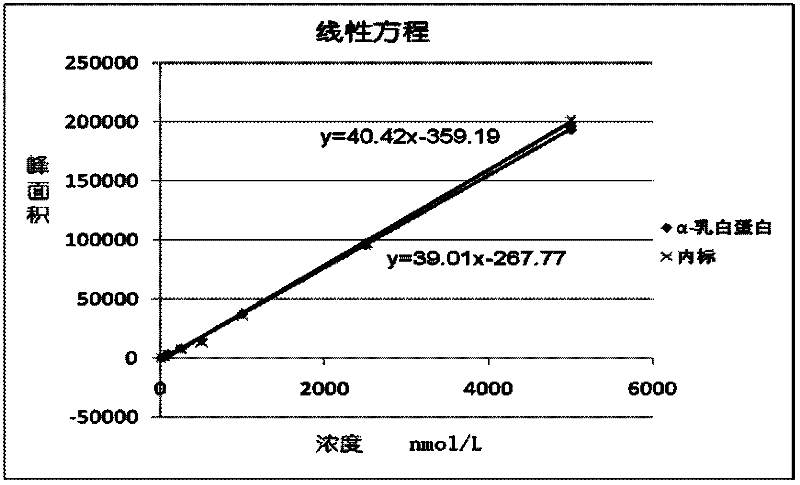
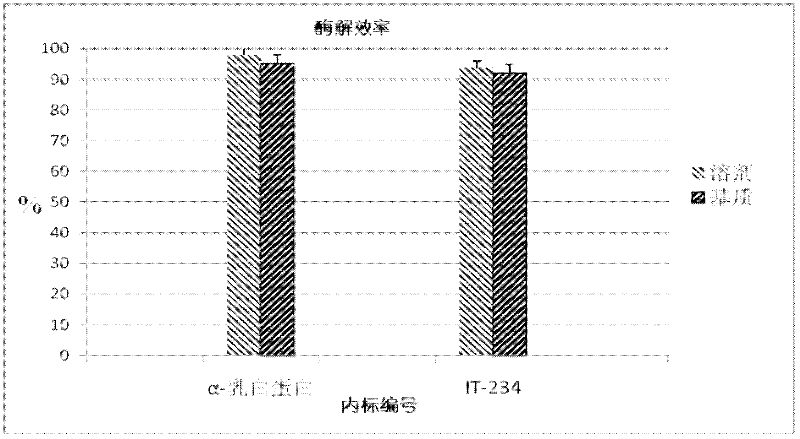
Quantitative detection method for bovine alpha-lactalbumin
InactiveCN102590413AEasy to operateGuaranteed reproducibilityComponent separationRelative standard deviationReversed-Phase Liquid Chromatography
The invention relates to a quantitative detection method for thermal-denaturation and non-denaturation bovine alpha-lactalbumin in milk and milk products by applying an enzymolysis-liquid chromatography and mass spectrometry combination technology. The quantitative detection method comprises the steps as follows: taking a certain amount of milk or milk samples, dissolving and diluting the milk or milk samples in water to obtain solution with total protein content being about 1mg / mL; after volume metering, correctly sucking 500 mu L, adding an internal standard substance, reacting disulfide bond with dithiothreitol (DTT), alkylating to protect sulfydryl produced in reaction by iodoacetamide (IAA), and then conducting constant-temperature and constant-time enzymolysis with trypsin; and separating enzymolysis products by reversed phase liquid chromatography, conducting detection with a mass spectrum multiple reaction monitoring (MRM) manner, and calculating the result by an internal standard method. The quantitation limit of the method is 0.001g / 100g; when adding amount is 0.2, 1.7 and 5.0g / 100g, the recovery rate is 98.9-110.8% (n is equal to 6) and repeatability: RSD (Relative Standard Deviation) is smaller than 7.6%; and the quantitative detection method can be applicable to the quantitative detection of samples with different contents of bovine alpha-lactalbumin.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
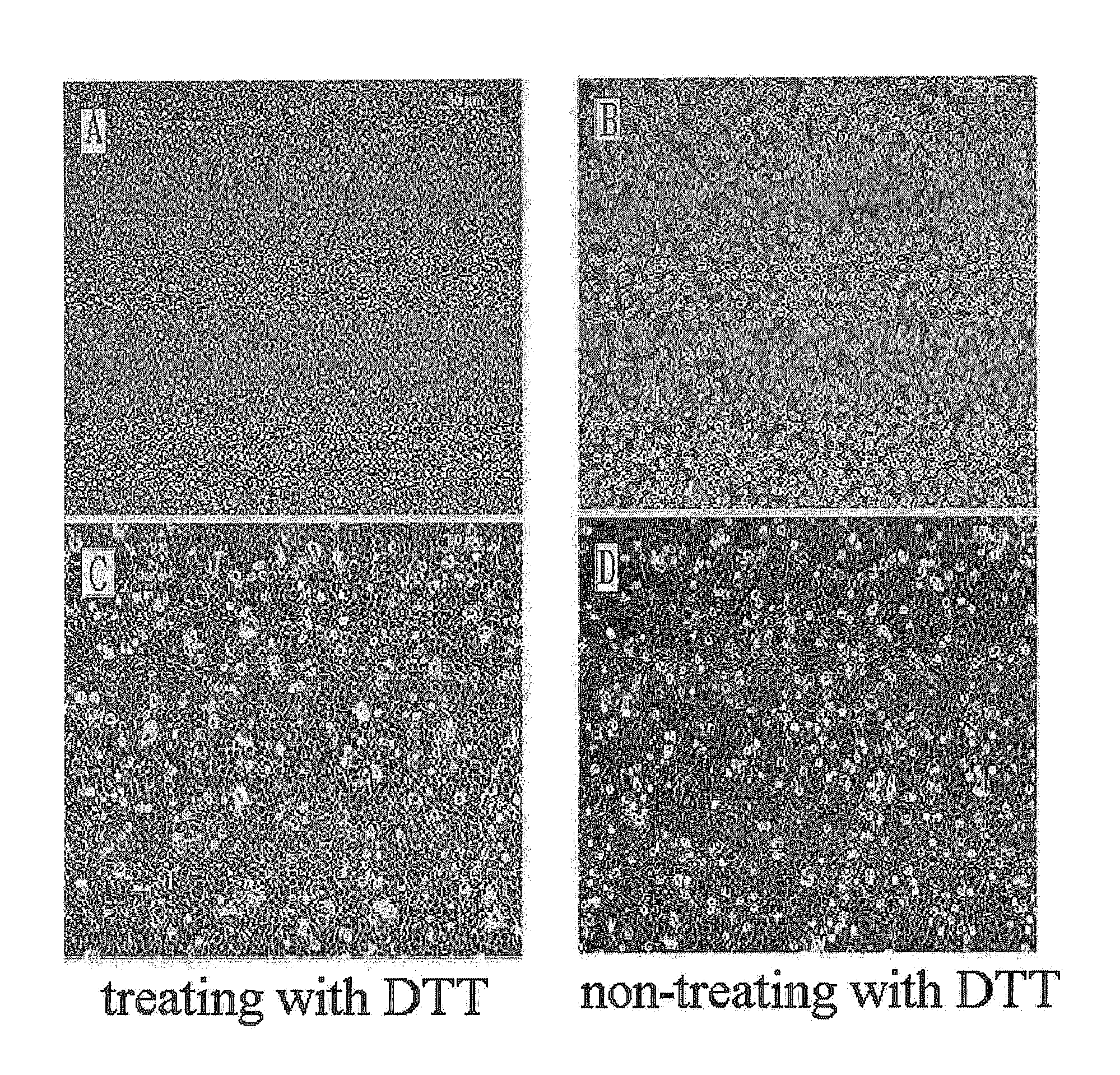
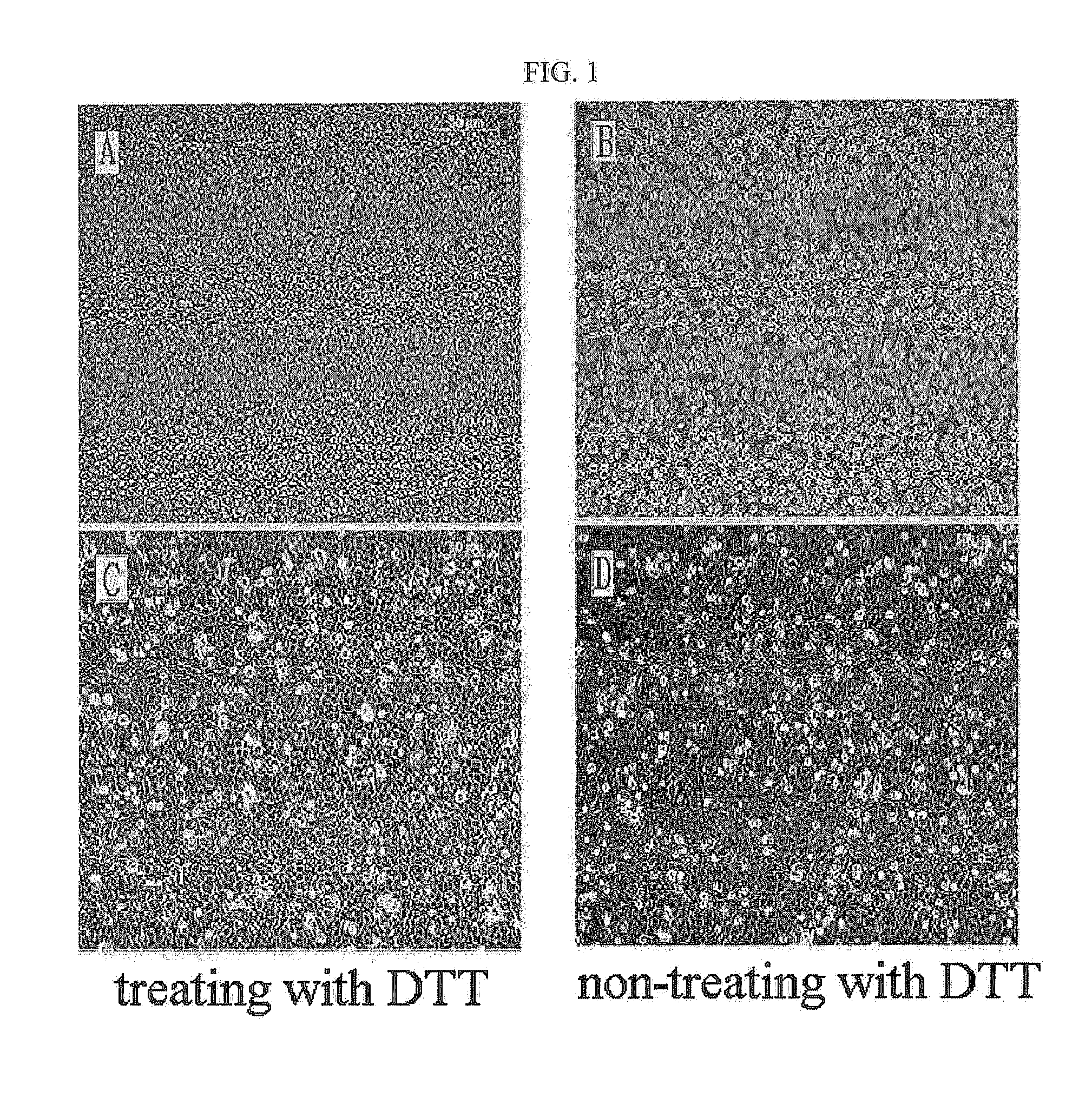
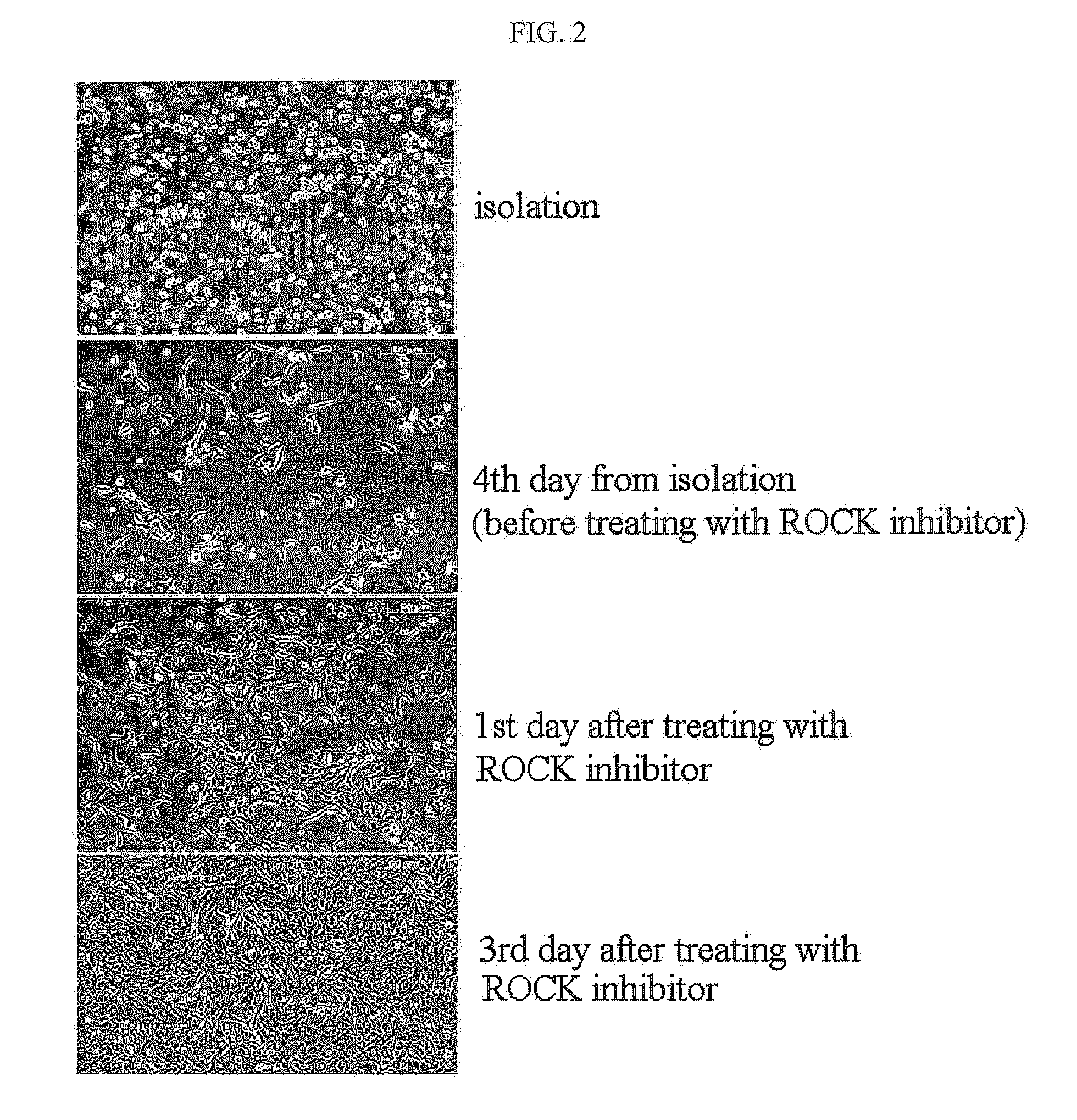
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Method and PCR (polymerase chain reaction) reagent kit for identifying DNA (deoxyribonucleic acid) barcodes of animal medicinal materials
InactiveCN105112525AGuarantee the success rate of amplificationEfficient amplificationMicrobiological testing/measurementBetainePolyethylene glycol
The invention provides a method and a PCR (polymerase chain reaction) reagent kit for identifying DNA (deoxyribonucleic acid) barcodes of animal medicinal materials. The PCR reagent kit comprises PCR enhancers. The PCR enhancers comprise bovine serum albumin, dithiothreitol, betaine and nonidet p40. The method and the PCR reagent kit for identifying the DNA barcodes of the animal medicinal materials have the advantages that low-abundance traditional Chinese medicine samples with difficulty in amplification, particularly, samples with difficulty in identifying DNA barcodes, such as samples of buffalo horns, antelope horns, tortoise shells, carapax trionycis, pangolins and snake slough, can be effectively amplified, accordingly, the amplification success rate which is close to 100%, of DNA barcode identification techniques can be guaranteed, and DNA barcode techniques can be widely applied.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
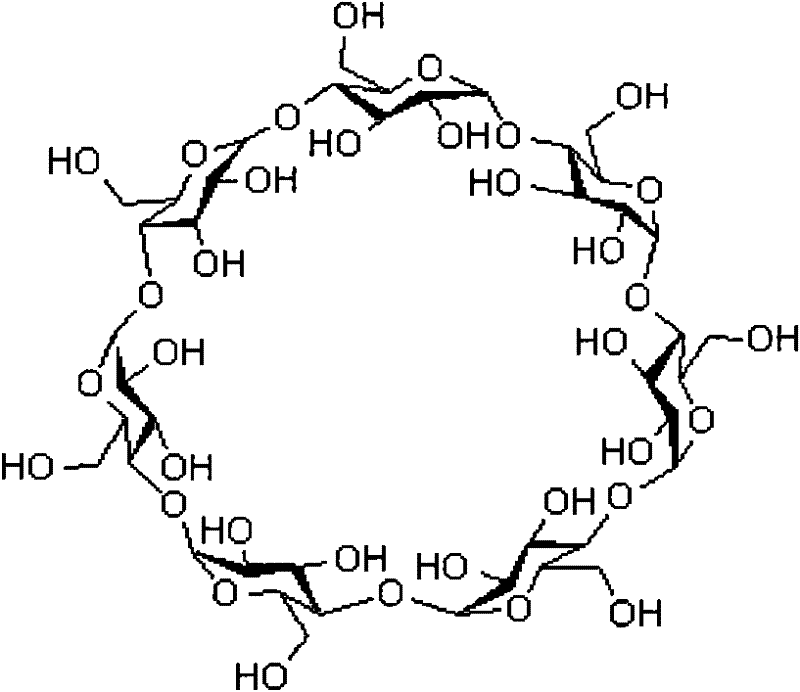
Method for refining sugammadex sodium
The invention discloses a method for re-crystallizing, refining and purifying sugammadex sodium. The method comprises the following steps: adding a protective agent to crude sugammadex sodium, and performing re-crystallizing under the protection of an inert gas to obtain pure sugammadex sodium, wherein the protective agent is selected from one or a mixture of two or more of mercaptoethanol, mercaptoacetate, mercaptoacetate ester, mercaptopropionate, mercaptopropionate ester, glutathione, cysteine, cystamine, dithioerythritol, dithiothreitol, trisubstituted organophosphorus compounds and trisubstituted organophosphorus compound salts. The method has the advantages of simplicity in operation, high product purity, good economy, and suitableness for industrial production.
Owner:王炳永
Swab eluting solution with sample preservation and inactivation functions
ActiveCN107227306ALow costImprove performanceMicrobiological testing/measurementDNA preparationClelands ReagentIsothiocyanate
The invention discloses a swab eluting solution with sample preservation and inactivation functions. The swab eluting solution is prepared from guanidinium isothiocyanate, ethylenediamine tetraacetic acid, a buffering solution, dithiothreitol, a surfactant, glass beads or polypropylene plastic beads and de-ionized water, wherein the contents of the components in the eluting solution are as follows: 20 weight / volume percent to 55 weight / volume percent of the guanidinium isothiocyanate, 1mM to 25mM of the ethylenediamine tetraacetic acid, 20mM to 100mM of the buffering solution, 1 weight / volume percent to 5 weight / volume percent of the dithiothreitol, 1 percent to 5 percent of the surfactant and 3 to 10 glass beads or polypropylene plastic beads. The prepared eluting solution is low-cost and stable in performance; the prepared eluting solution can be used for preserving DNA / RNA (Deoxyribonucleic Acid / Ribonucleic Acid), and elution and normal-temperature preservation and transportation of a swab sample are realized; pathogens can be inactivated, so that the swab eluting solution has a sample inactivation effect, and risks of exposed infection in a laboratory can be greatly reduced; the guanidinium isothiocyanate which is used in the eluting solution is a common chemical component for extracting nucleic acid, so that the subsequent extraction of the nucleic acid is not influenced.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Method for inhibiting degradation of decarburization amine absorbent
ActiveCN103007687AAdd lessImprove antioxidant capacityProductsOrganic compound preparationAntioxidantOxidation resistant
The invention relates to a method for inhibiting degradation of a decarburization amine absorbent. Specifically, certain amount of antioxidant is added into the amine absorbent to inhibit degradation of the amine absorbent. The used antioxidant can be xylitol, threitol, dithiothreitol, erythritol, ethylene glycol, butanediol and other structurally similar alcohol compounds. By means of the principles of interrupting an oxidation reaction chain and decomposing an oxidative degradation product, the antioxidant can achieve significant antioxidant effects, and can be used under the circumstances of small adding amount, high temperature and wide oxygen content, thus greatly inhibiting degradation of amine water solution. Stability of a recovery solvent and continuous operation of separation equipment are ensured, the degree of corrosion caused by an absorption solvent on equipment can be reduced, and meanwhile the absorbent loss is also reduced. The method can be used for antioxidation of amine absorption solvents applied in capture and separation of carbon dioxide (CO2) from natural gas, shift gas, synthetic gas, flue gas and other industrial feed gas.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for extracting RNA in gymnosperm tissue
InactiveCN101492485AHigh purityQuality improvementSugar derivativesSugar derivatives preparationRNA extractionPolyphenol
The invention discloses a method for extracting RNA from gymnosperm tissues. The method includes the following steps: 1) extracting buffer solution by RNA to treat plant materials; 2) protein removal: extracting by equal-volume chloroform / isoamylol for twice; 3) using LiCl to deposit RNA and SSTE to dissolve back RNA; 4) glycan removal: adding absolute ethyl alcohol and potassium acetate to the dissolved solution acquired in step 3) and depositing glycan in ice bath; 5) extracting chloroform / isoamylol again; 6) depositing RNA and DEPC H2O again to dissolve back RNA. In order to remove DNA, the following steps can be also conducted: a) using DNase without RNase pollution to remove DNA; b) using chloroform / isoamylol to extract and remove DNase; (c) repeating step 6). Wherein, RNA extract and buffer solution contains 100mmol / L Tris HCl(pH 8.0), 2g / 100mlCTAB, 2g / 100mlPVP, 2mol / LNaCl and 25mmol / L EDTA and is added with dithiothreitol with final concentration of 10mmol / L and 2 percent (volume percentage) of beta-mercaptoethanol. Therefore, the invention solves the interference of glycan and polyphenol and the like and acquires high-purity RNA.
Owner:BEIJING FORESTRY UNIVERSITY +1
Preservative composition of blood DNA
The invention provides a preservative composition of blood DNA. The preservative composition of blood DNA comprises, by weight, 20 to 50 parts of a preservative, 5 to 15 parts of an anticoagulant, 0.5 to 5 parts of a nuclease inhibitor, 1 to 5 parts of a metabolic inhibitor and 40 to 70 parts of water, wherein the nuclease inhibitor comprises one or more of diatomite, bentonite, ethyl alcohol, formamide, vanadyl-ribonucleoside complex, dithioerythritol, diethyl pyrocarbonate, proteinase-K, heparin, beta-mercaptoethanol, hydroxylamine-O-copper ions, ammonium sulfate, dithiothreitol, cysteine, and 4-formylbenzoic acid and oxovanadium Schiff base complex.
Owner:上海迅伯生物科技有限公司 +1
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay kit and detection method for grass carp reoviruses
InactiveCN102703608AEfficient detectionStrong specificityMicrobiological testing/measurementMicroorganism based processesWater bathsBetaine
The invention discloses a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay kit and a detection method for grass carp reoviruses (GCRV), and belongs to the technical field of the detection of fish viruses. The RT-LAMP assay kit for the grass carp reoviruses comprises the following ingredients: 1*ThermoPol Reaction Buffer, Bacillus stearothermophilus deoxyribonucleic acid (BstDNA) polymerase, deoxyribonucleotide triphosphate (dNTPs), AMV reverse transcriptase, outer primers (F3 and B3), inner primers (FIP and BIP), Betaine, MgCl2, dithiothreitol (DTT) and 1,000*SYBR Green I. The assay kit has the characteristics of simpleness, convenience, quickness and high specificity and sensitivity; GCRV-104 in a sample can be accurately detected within 2 hours only by using a water bath kettle or metal bath; and the assay kit can detect grass carp tissues infected by the GCRV-104 and cells (such as cytokine-induced killer (CIK) cells) infected by the GCRV-104, and is applicable to the on-site rapid detection of the GCRV-104.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
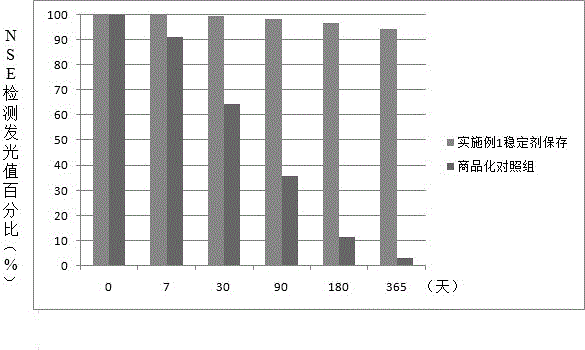
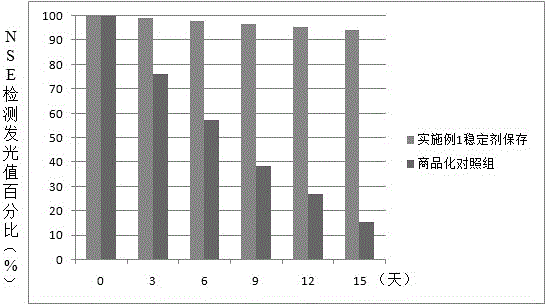
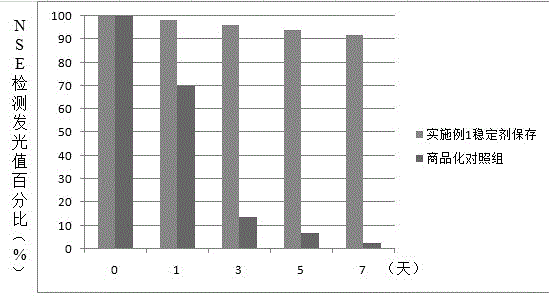
Neuron-specific enolase stabilizer and preparation method thereof
InactiveCN106568976AImprove overall lifespanReduce manufacturing costBiological testingFreeze-dryingBovine serum albumin
The invention relates to a neuron-specific enolase stabilizer. The stabilizer is prepared from a Tris.HCl buffer solution, NaCl, sucrose, trehalose, casein, bovine serum albumin, PEG6000, lysine, disodium ethylene diamine tetraacetate, dithiothreitol, glycerin, Tween-20, Triton X-100, aprotinin with the final concentration of 100-300 KIU / mL, and Proclin 300, and above substances are uniformly mixed in Millipore ultrapure water used as a solvent, is filtered by a 0.22 [mu]m filter membrane, and finally is preserved at 0-10 DEG C. The stabilizer greatly improves the preservation stability of neuron-specific enolase, prolongs the shelf life of a kit, simplification of the production technology and the doctor's detection operation process of a neuron-specific enolase detection kit, improvement of the metamorphism resistance in the preparation, transportation and storage process of the neuron-specific enolase, and reduction of inter-assay variation caused by a freeze-drying technology.
Owner:JIANGSU FLON BIOTECH
A method of promoting keratin dissolution and enhancing strength of a keratin material
InactiveCN106867000AHigh mechanical strengthLow cytotoxicityMonocomponent protein artificial filamentArtifical filament manufactureSolubilityFiber
The invention relates to a method of promoting keratin dissolution and enhancing strength of a keratin material. The keratin relates to water-insoluble keratin or keratin the solubility of which in water is low, and is characterized in that the disulfide bond content of the keratin is high. The method of promoting dissolution of the water-insoluble keratin includes adding the keratin into an aqueous solution, and adding a certain amount of a reductant at the same time to break disulfide bonds to form free mercapto groups, thus promoting keratin dissolution. The reductant includes cysteine, dithiothreitol, glutathione, mercaptan and sodium pyrosulfite, or keratin or peptides with reducibility, or other compounds capable of inducing disulfide bond breakage and formation of the free mercapto groups, such as proteins or peptides with reducibility. The method is also characterized in that the dissolved keratin comprises the free mercapto groups so that the dissolved keratin can be subjected to disulfide bond crosslinking again under oxidation conditions, thus significantly improving mechanical strength of the keratin material. The keratin material can includes keratin hydrogel, film, sponge, fibers, and a plurality of other forms.
Owner:SUZHOU BAIRUI BIOLOGICAL SCI & TECH CO LTD
Liquid-based cell preservation solution
InactiveCN105409925ABrightly dyedLess inflammatory cellsDead animal preservationPotassiumCervical mucus
The present invention relates to pathological examination, especially to a liquid-based cell preservation solution used in pathologically examining cells such as human cervical mucus, sputum, urine, thoracic liquid, and tracheal mucus. The liquid-based cell preservation solution is characterized by being prepared from alcohols, a sodium phosphate buffer solution, disodium ethylenediamine tetraacetate, 0.08-0.12% sodium chloride, potassium chloride, formaldehyde, dithiothreitol, calcium acetate, magnesium acetate and the like. The cell preservation solution cannot only maintain the cell structure stability and reduce the agglomerated precipitation of the cell mucus and the cell breakage loss, but also enables the cells easily to be stained, improves the clarity of the cell sheets, and facilitates smooth performance of the examination work. Moreover, the cell preservation solution is low in cost and easy to promote and use.
Owner:孝感宏翔生物医械技术有限公司
Kit and method for constructing DNA library
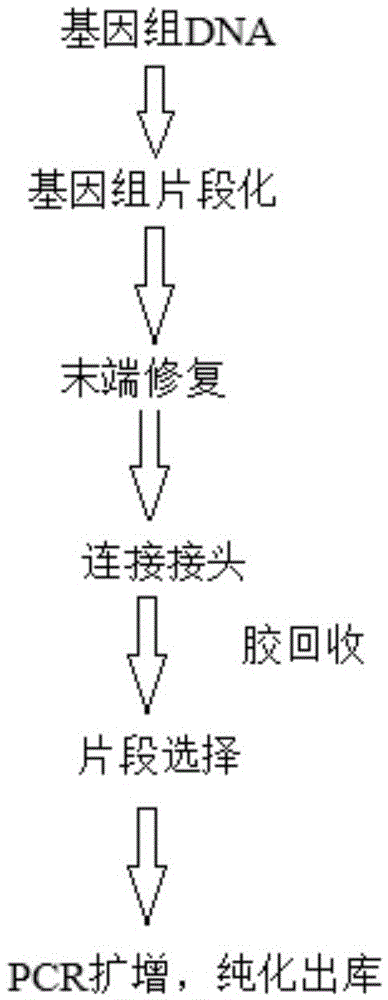
ActiveCN105603535AAchieve end repairAchieve plus ALibrary creationT4 polynucleotide kinasePolymerase L
The invention provides a kit and method for constructing a DNA library. The kit comprises an enzymic reagent, a buffer solution and a joint combination, wherein the enzymic reagent comprises a first enzyme mixed liquid, T4DNA ligase and a second enzyme mixed liquid, and the first enzyme mixed liquid is formed by mixing T4 Polynucleotide Kinase, T4DNA polymerase, a Klenow fragment and rTaq enzyme; the second enzyme mixed liquid is a 2X KAPA HiFi HotStart pre-mixed solution; the buffer solution comprises a first buffer solution and a second buffer solution, and the first buffer solution is formed by mixing a 10X T4DNA ligase buffer solution, dNTP mixed liquor and ATP; the second enzyme mixed liquid is formed by mixing ATP, Tris-HCL, MgCl2, PEG8000, Tween 20 and dithiothreitol; the joint combination comprises a joint sequence group and a tag sequence group. According to the kit, the library construction cost can be reduced and the amplification efficiency is improved.
Owner:BEIJING NOVOGENE TECH CO LTD
Gene diagnosis and detection core reagent transportable at normal temperature and of high sensitivity and high specificity
InactiveCN102242188AImprove stabilityThe result is stableMicrobiological testing/measurementGlycerolBovine serum albumin
The invention relates to a gene diagnosis and detection core reagent transportable at normal temperature and of high sensitivity and high specificity. The reagent comprises reaction buffer, deoxyribonucleoside triphosphate (DNTP), heat resistant DNA polymerase, glycerin, bovine serum albumin (BSA), dithiothreitol (DTT) and tween-20. The reagent is characterized in that: proper amounts of polysaccharide and disaccharide are mixed into the core reagent; the reaction buffer of the reagent is composed of 5mM-50mM of Tris-HCL, 20mM-500mM of KCL, 1.0mM-5mM of MgC12 and 20mM-200mM of (NH4)2SO4; meanwhile 50uM-300uM of DNTP, 0.01U / ul-0.2U / ul of heat resistant DNA polymerase, 1mM-15mM of polysaccharide, 2M-100M of disaccharide, 3%-30% of glycerin, 10-300ug / ml of BSA, 0.5-10mM of DTT and 0.005-0.05v / v of tween-20 are mixed.
Owner:SHANGHAI XINGHAN SCI&TECH
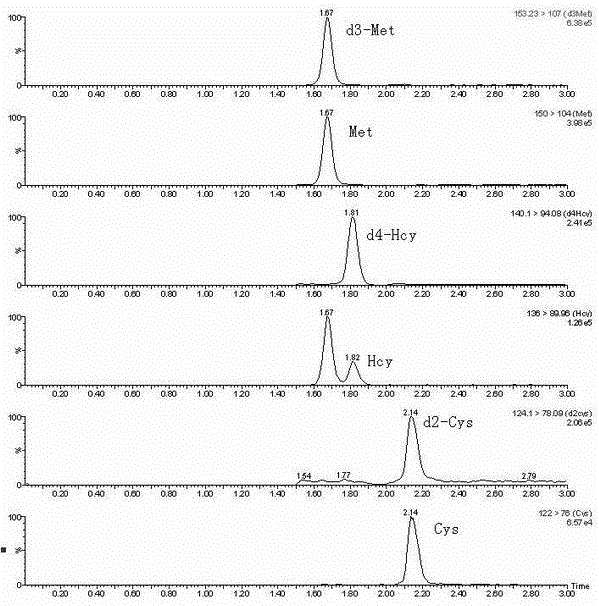
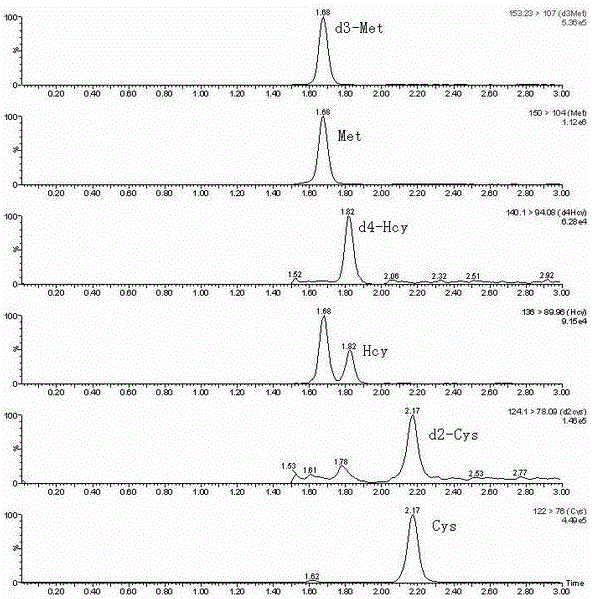
Liquid chromatography tandem mass spectrum method for detecting sulphur amino acids in blood plasma using non-derivation method
InactiveCN106841488AGuaranteed accuracyThe result is accurateComponent separationCysteine thiolateIsotope
The invention discloses a liquid chromatography tandem mass spectrum method for detecting sulphur amino acids in blood plasma using a non-derivation method and belongs to the field of biochemical analysis and detection. The method comprises the steps that the blood plasma is added to an isotope internal standard solution, and a mixed solution is mixed evenly, then, dithiothreitol (DTT) is added to restore, and supernatant with the sulphur amino acids is obtained after protein precipitation treatment. A sample is pretreated by the adoption of the non-derivation method, the method is simple, the operation is easy, and the sample pretreatment procedures are greatly simplified; the interference for detection by matrix is reduced by the isotope internal standard, and the accuracy of homocysteine, cysteine and methionine detection is ensured. According to the liquid chromatography tandem mass spectrum method for detecting the sulphur amino acids in the blood plasma using the non-derivation method, an LC-MS / MS method is used for detecting the sulphur amino acids in the blood plasma, a multiple-reaction detection MRM scanning mode is adopted, cross interference between (i) d ( / i) (i) 3 ( / i) -methionine and homocysteine (136> 90 passage) exists, base lines of the cross interference are separated by elution conditions of a chromatography gradient, and the accuracy of the detection is ensured. The method is high in sensitivity, strong in specificity and accurate in result.
Owner:LIAONING RUNSHENG KANGTAI BIOMEDICAL TECH CO LTD +1
PCR amplification system and PCR amplification method for high GC content gene
The invention discloses a PCR amplification system and a PCR amplification method for high GC content gene, belonging to the technical field of biotechnology; the PCR amplification system comprises DNA polymerase, MgCl2, dNTP, upstream primer, downstream primer, DNA template and double distilled water; and the invention is characterized in that the PCR amplification system also comprises tri(hydroxymethyl)aminomethane hydrochloride, dithiothreitol, bovine serum albumin and additive. The PCR amplification technology in the invention can amplify target genes containing over 80% of GC, can amplify high GC content target genes with a length up to 1.5Kb and can successfully amplify template genes with different degrees of complexity and different sources such as people, animals, plants and microorganisms; in addition, the PCR amplification technology has less operating steps, short operating time, simple instruments and devices, thus being generally applicable to the scientific research and medical diagnosis.
Owner:陈学军
Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor
InactiveUS20050260712A1Procedure of process can be improvedReduce processing timeSugar derivativesBacteriaPhenylalanine dehydrogenaseDipeptidyl peptidase
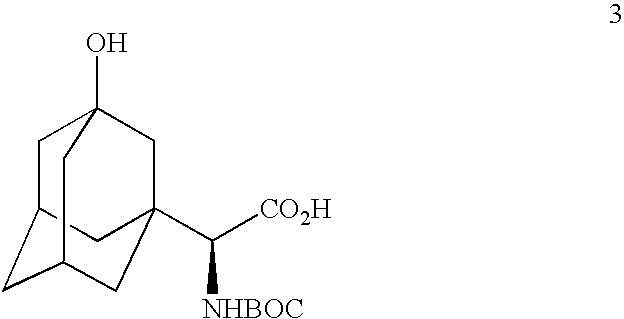
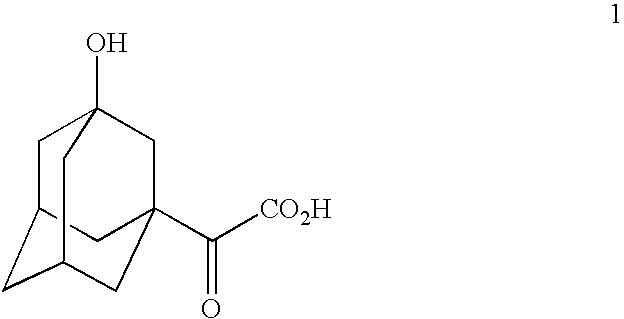
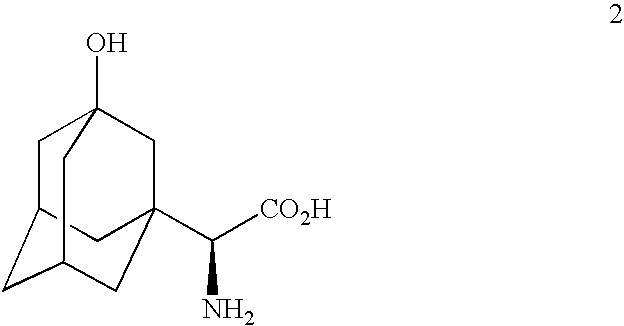
A process for production of cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV is provided which employs a BOC-protected amine of the structure prepared by subjecting an acid of the structure to reduce amination by treating the acid with ammonium formate, nicotinamide adenine dinucleotide, dithiothreitol and partially purified phenylalanine dehydrogenase / formate dehydrogenase enzyme concentrate (PDH / FDH) and without isolating treating the resulting amine of the structure 2 with di-tert-butyl dicarbonate to form the BOC-protected amine.
Owner:ASTRAZENECA AB
Method of simultaneously extracting animal DNA (Deoxyribonucleic Acid) virus and RNA (Ribonucleic Acid) virus nucleic acid in blood serum and double swabs
The invention discloses a method of simultaneously extracting animal DNA (Deoxyribonucleic Acid) virus and RNA (Ribonucleic Acid) virus nucleic acid in blood serum and double swabs. The method comprises the following steps of: carrying out lysis on a to-be-extracted substance by using guanidinium isothiocyanate lysate; adsorbing RNA by a silica gel membrane; removing impure protein by washing liquor I; removing impurities by washing liquor II; carrying out DEPC (Diethylpyrocarbonate) water-washing to remove nucleic acid, wherein the guanidinium isothiocyanate lysate comprises 3M-7M guanidinium isothiocyanate, 0.6%-1.0% TriTon-100, 30mM-50mM Tris-Cl, 5mM-15mM DTT (DL-Dithiothreitol), 60 mu g / mL-90 mu g / mL protease K, 10mM-30mM EDTA (Ethylene Diamine Tetraacetic Acid), and PH of the guanidinium isothiocyanate lysate is 4.3-4.6; the washing liquor I comprises 5M-6M guanidine hydrochloride, 53%-59% absolute ethyl alcohol, 70-90 mu g / mL protease K, and PH of the washing liquor I is 6.4-6.6; the washing liquor II comprises 70%-80% alcohol. The method disclosed by the invention has the advantages of being simple in extracting process, short in period, low in cost, and capable of simultaneously extracting RNA virus and DNA virus nucleic acid in an animal blood serum sample and a double-swab sample.
Owner:安徽华卫集团禽业有限公司
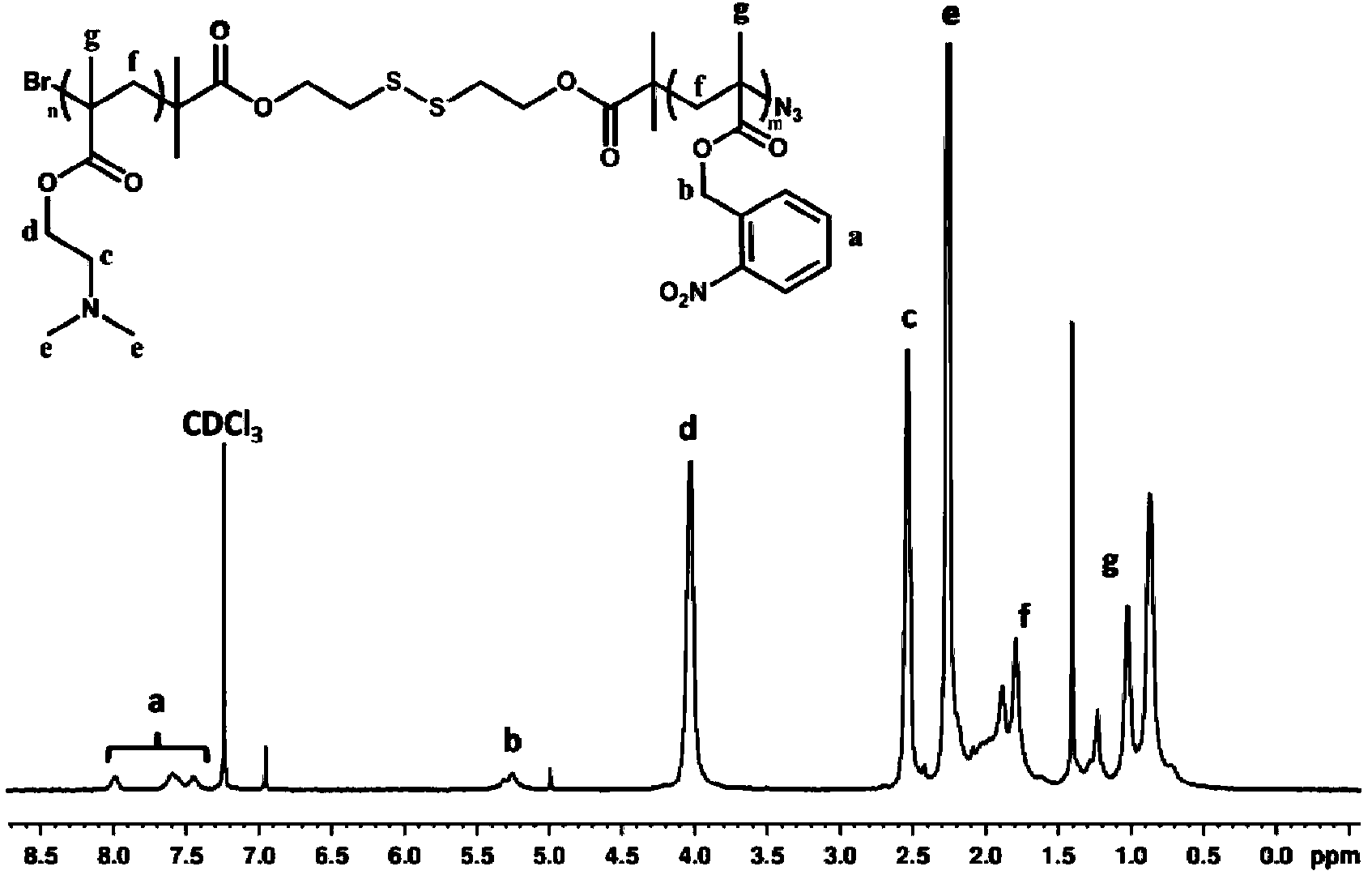
Preparation method and application of quadruple-responsiveness block micelle
The invention discloses a preparation method and an application of a novel quadruple-responsiveness (temperature, pH, reduction and photo-response) block polymer micelle. The preparation method of the novel quadruple-responsiveness block polymer micelle comprises the following steps: enabling an atom transfer radical polymer to react for two times so as to prepare a poly(2-nitryl benzyl methyl acrylate)-block-poly(dimethylaminoethyl methacrylate) (PNBM-SS-PDMAEMA) amphiphilic block copolymer, wherein the polymer is capable of self-assembling in water to form a nano-micelle and also capable of loading hydrophobic micromolecules (for example, nile red). The structure of the self-assembled micelle is capable of obviously changing in the presence of ultraviolet radiation, reducing agent dithiothreitol, temperature regulation and pH stimulation; and different synergetic stimulations are capable of regulating dynamic release processes of guest molecules. The polymer micelle has temperature response, pH response, reduction response and photo-response and also has a wide application prospect in the field of controlled release.
Owner:UNIV OF SCI & TECH BEIJING
Enzymatic catalysis crosslinking reduction-responsive hyaluronic acid microgel and preparation method thereof
InactiveCN105969825AQuick releaseFast curing ratePharmaceutical non-active ingredientsFermentationTumor targetBiocompatibility Testing
The invention discloses enzymatic catalysis crosslinking reduction-responsive hyaluronic acid microgel and a preparation method thereof. The hyaluronic acid microgel is prepared by taking sulfhydrylation hyaluronic acid as a raw material, taking horseradish peroxidase as a catalyst, taking tyramine hydrochloride as an enzymatic catalysis reaction substrate and combining the method that horseradish peroxidase is catalytically crosslinked with sulfydryl to generate a disulfide bond through an inverse emulsion method. The disulfide bond crosslinking structure in the microgel can be broken in the presence of reductive substances such as dithiothreitol, reductive glutathione hormone and L-cysteine, and then the good reduction responsiveness is given to the microgel. The hyaluronic acid microgel can achieve controlled release on the reduction responsiveness of loaded doxorubicin hydrochloride and can be applied to controlled release of tumor-targeted drugs. According to the preparation method, a crosslinking agent does not need to be additionally added into an inverse emulsion system to cure the microgel, the toxicity influence of the crosslinking agent is avoided, the biocompatibility of the microgel is guaranteed, the reaction conditions are mild, and the technological processes are simple.
Owner:NANCHANG UNIV
Method for extracting total RNA (Ribonucleic Acid) from woody mangrove plants and special extracting solution thereof
The invention discloses a method for extracting total RNA (Ribonucleic Acid) from woody mangrove plants and special extracting solution thereof. The extracting solution provided in the invention comprises Tris-HCl, EDTA (Ethylene Diamine Tetraacetic Acid), NaCl, SDS (Sodium Dodecyl Sulfonate), DTT (Dithiothreitol), beta-mercaptoethanol and water, wherein the final concentration of the Tris-HCl is 75-125 mmol / L, the final concentration of the EDTA is 400-600 mmol / L, the final concentration of the NaCl is 400-600 mmol / L, the final concentration of the SDS is 1%-3% (in weight percent), the final concentration of the DTT is 3-8 mmol / L, and the final concentration of the beta-mercaptoethanol is 1%-3% (in weight percent); and the pH value is 8-9. The method provided in the invention is used for extracting the total RNA from the woody mangrove plants by using the extracting solution. The RNA extracted by the method can be directly used for synthesizing cDNA (complementary Desoxyribonucleic Acid) and carrying out molecular biology operations, such as library establishment, molecular clone and the like.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
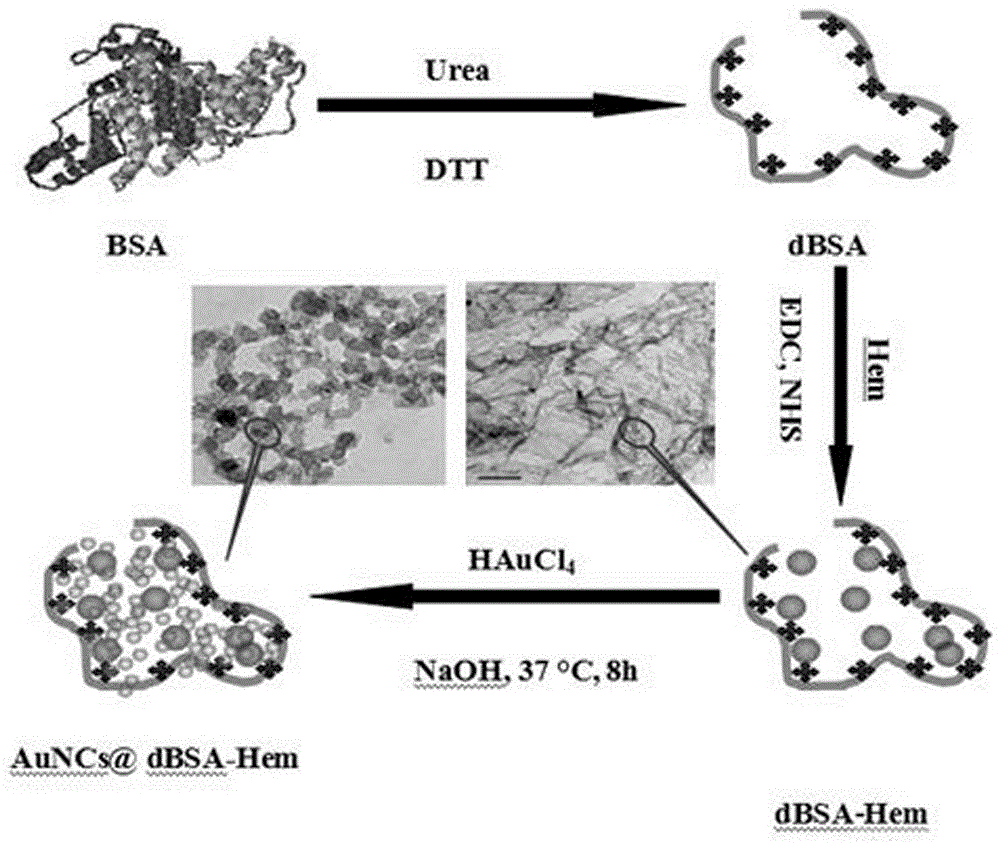
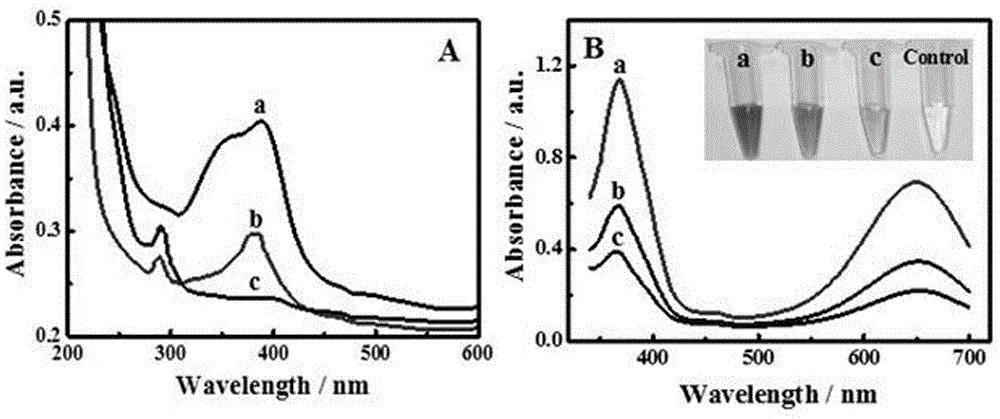
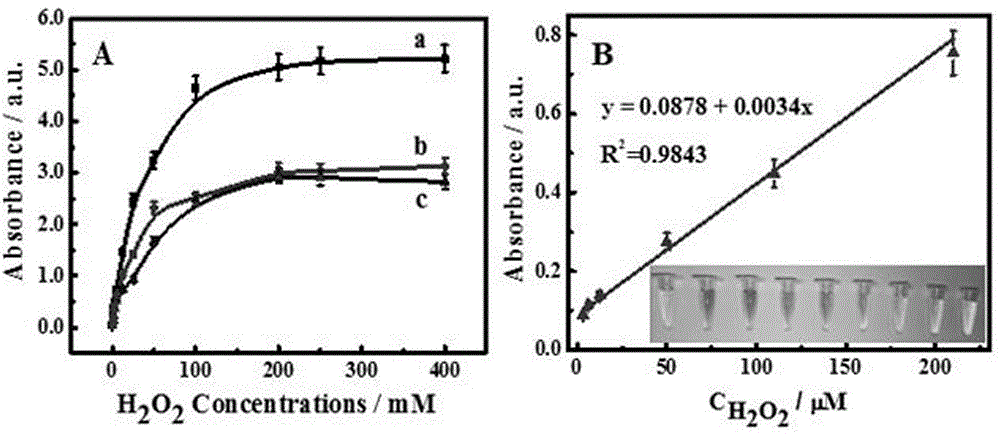
Application and preparation method of protein mimic enzyme based on heme and gold nanoclusters
ActiveCN105675598ASolving activitySolution insoluble in waterMaterial analysis by observing effect on chemical indicatorMaterial analysis by electric/magnetic meansHeminBovine serum albumin
The invention provides application and a preparation method of a protein mimic enzyme based on heme and gold nanoclusters.The method includes: using carbamide for denaturation of bovine serum albumin, using 1,4-dithiothreitol for recovering the bovine serum albumin to obtain sulfydryl-containing chain bovine serum albumin, crosslinking with hemin to obtain sulfydryl-containing chain bovine serum albumin and hemin scaffold composite, and taking the composite as a stabilizer and a reducing agent to synthesize the gold nanoclusters to further obtain the protein mimic enzyme based on the heme and the gold nanoclusters.The protein mimic enzyme prepared according to the method has the advantages of low cost, high catalytic activity, high stability and the like.The defects of low catalytic activity, insolubility in water, proneness to clustering in water solutions, proneness to structural damages in oxidation media and the like of the heme are overcome, and application range of the heme is widened.In addition, the protein mimic enzyme is application to quick and high-sensitivity detection of H2O2 content in common foods, avoids complex photoelectric instruments in a detection process and is simple and easy in operation, thereby being expected to be widely applied to the field of biocatalysis.
Owner:QUFU NORMAL UNIV
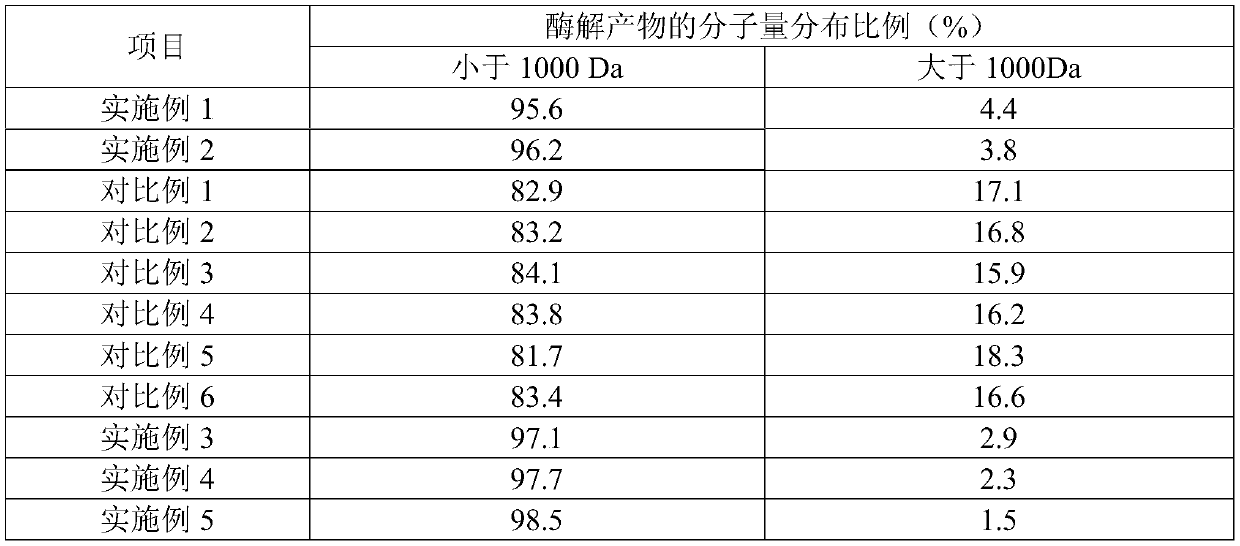
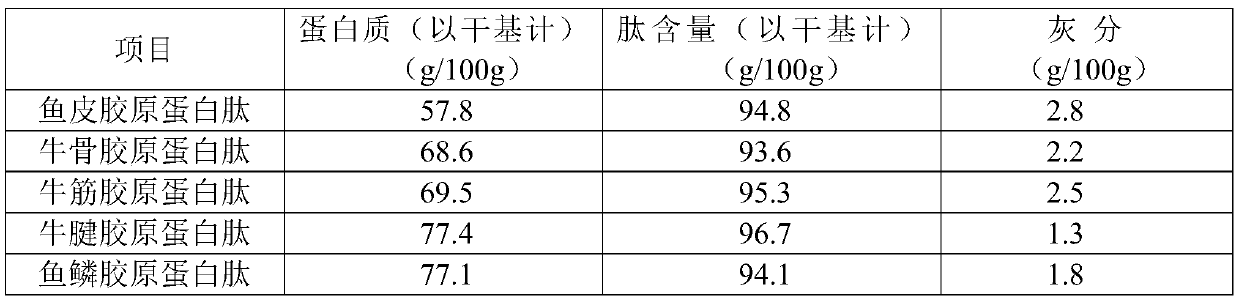
Collagen peptide, production method, production device and application thereof
InactiveCN110024901ASmall molecular weight and concentratedLow in free amino acidsProtein composition from fishAnimal proteins working-upNeutral proteaseMoisture
The present invention belongs to the field of food-derived peptides and discloses a collagen peptide, a production method, a production device and an application thereof. The production method of thecollagen peptide comprises steps of coarse collagen solution heating, cooling, high-efficiency compound enzyme preparation enzymatic hydrolyzing, membrane filtering, concentrating, ultra-high pressurehomogenizing, and ultra-high temperature instantaneous sterilizing and drying. The high-efficiency compound enzyme preparation enzymatic hydrolyzing uses a compound enzyme preparation comprising compound protease, alkaline protease, neutral protease, flavor protease, animal proteolytic enzyme and an enzyme activator, and the enzyme activator is dithiothreitol and / or beta-mercaptoethanol. The provided method does not need to adjust pH value of the enzymatic hydrolyzing, does not need to control enzymatic hydrolyzing temperatures, is short in enzymatic hydrolyzing time and small in enzyme addition amount, and enables the enzymatic hydrolyzed product to be low and concentrated in molecular weight. The prepared collagen peptide powder is free of fishy smell, extremely excellent in water resolubility and also not liable to absorb moisture and agglomerate during storage process, and can be used in solid beverages, effervescent tablets, chewable tablets, health-care foods and feature-beautifying and skin-protecting products.
Owner:JIMEI UNIV
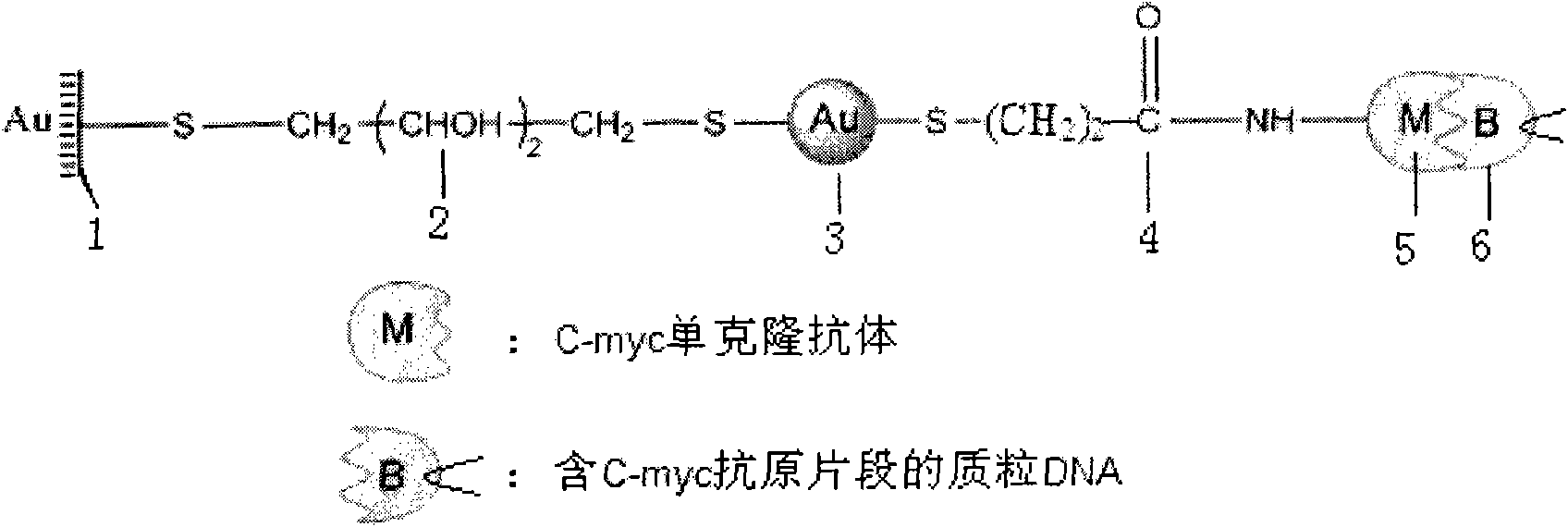
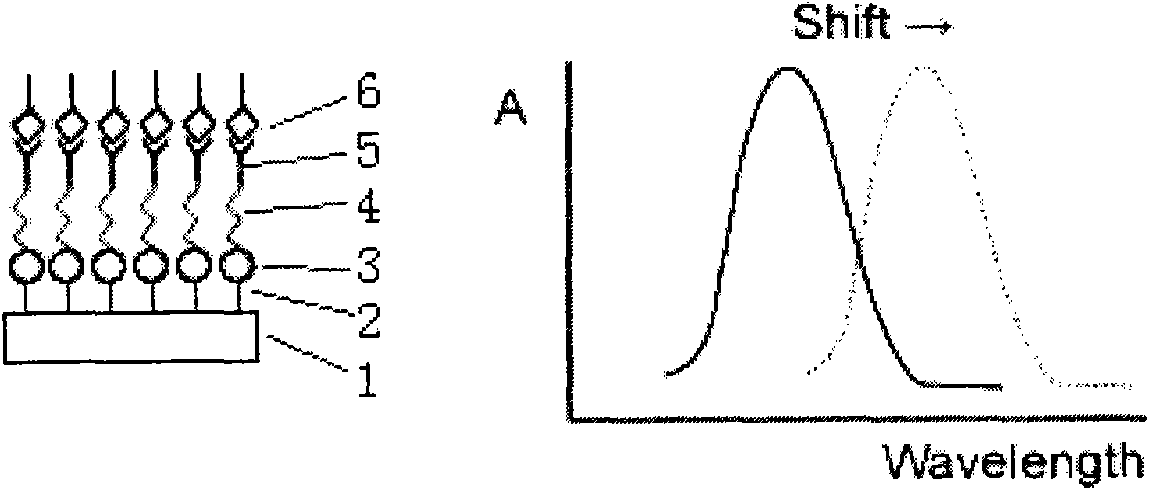
LSPR (Localized Surface Plasmon Resonance) sensing chip for detecting plasmid DNA (deoxyribonucleic acid) containing oncogene C-myc antigen fragment
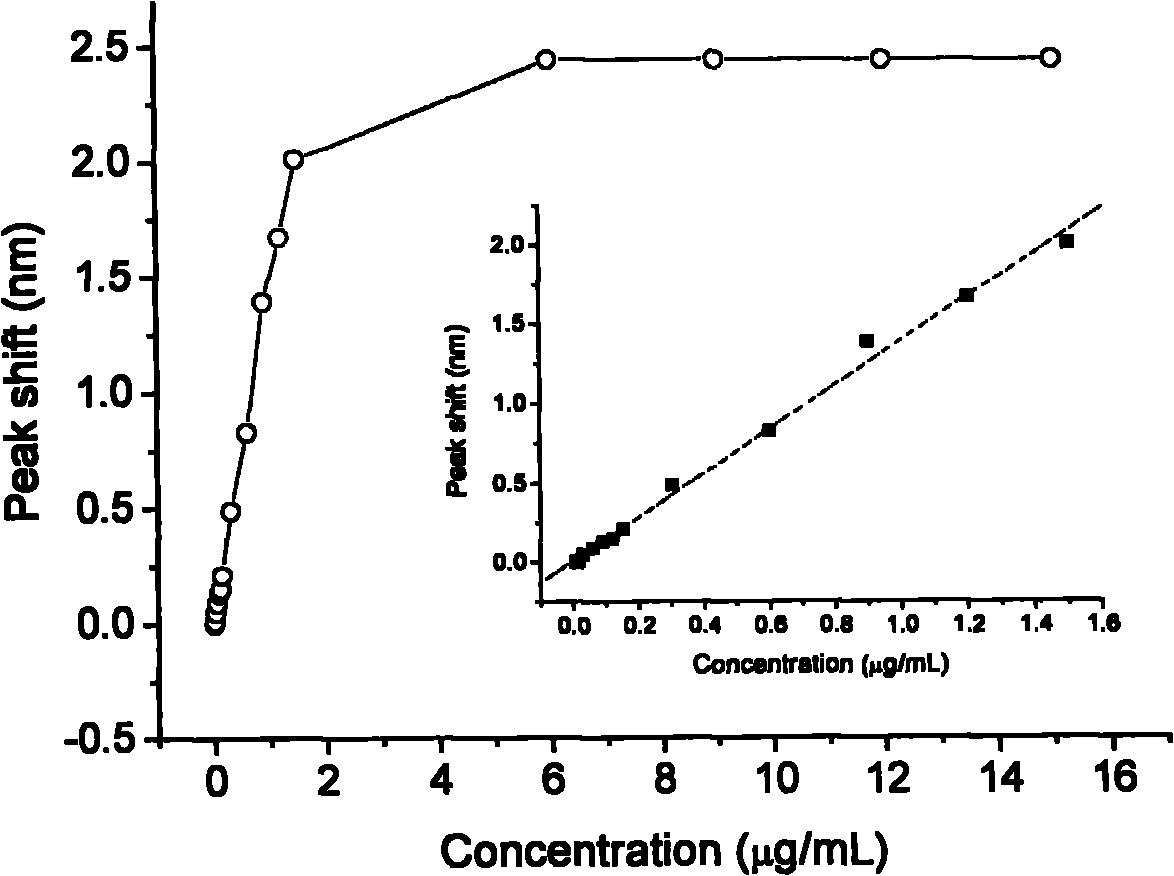
The invention provides an LSPR (Localized Surface Plasmon Resonance) sensing chip for detecting plasmid DNA (deoxyribonucleic acid) containing an oncogene C-myc antigen fragment on the basis of LSPR. The LSPR sensing chip is characterized in that one DTT (Dithiothreitol) unimolecule layer (2) is assembled on the surface of a gold film (1) of the LSPR sensing chip; a gold nanoparticle layer (3) is accessed; the surface of the gold nanoparticle layer (3) is furnished with a 3-mercaptopropionic acid unimolecule layer (4); then DMAP-EDC [4-Dimethylamino pyridine-1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide] activation ensures that the 3-mercaptopropionic acid unimolecule layer (4) is combined with a C-myc monoclonal antibody (5); and the immune response combination of the C-myc monoclonal antibody (5) and the plasmid DNA (6) containing the C-myc antigen fragment causes that the surface of the gold nanoparticle generates the displacement of a local surface plasma resonance absorption peak so as to detect the content of the plasmid DNA (6) containing the C-myc antigen fragment in canceration tissues. The invention has the beneficial effects that the LSPR sensing chip has the advantages of multiple-path detection, high sensitivity and good selectivity and repeatability, is simple and convenient to assemble and is quick to quantify. The LSPR sensing chip is superior to the LSPR sensing chip manufactured with the traditional chromatin immunoprecipitation (ChIP) method.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Universal procedure for refolding recombinant proteins
A universal folding method that has been demonstrated to be effective in refolding a variety of very different proteins expressed in bacteria as inclusion bodies has been developed. Representative proteins that can be dissolved and refolded in biologically active form, with the native structure, are shown in Table I. The method has two key steps to unfold and then refold the proteins expressed in the inclusion bodies. The first step is to raise the pH of the protein solution in the presence of denaturing agents to pH greater than 9, preferably 10. The protein solution may be maintained at the elevated pH for a period of up to about 24 hours, or the pH immediately decreased slowly, in increments of about 0.2 pH units / 24 hours, until the solution reaches a pH of about 8.0, or both steps used. In the preferred embodiment, purified inclusion bodies are dissolved in 8 M urea, 0.1 M Tris, 1 mM glycine, 1 mM EDTA, 10 mM beta-mercaptoethanol, 10 mM dithiothreitol (DTT), 1 mM reduced glutathione (GSH), 0.1 mM oxidized glutathione (GSSG), pH 10. The absorbance at 280 nm (OD280) of the protein solution is 5.0. This solution is rapidly diluted into 20 volumes of 20 mM Tris base. The resulting solution is adjusted to pH 9.0 with 1 M HCl and is kept at 4° C. for 24 hr. The pH is adjusted to pH 8.8 and the solution is kept at 4° C. for another 24 hrs. This process is repeated until the pH is adjusted to 8.0. After 24 hr at pH 8.0, the refolded proteins can be concentrated by ultrafiltration and applied to a gel filtration column for purification.
Owner:OKLAHOMA MEDICAL RES FOUND
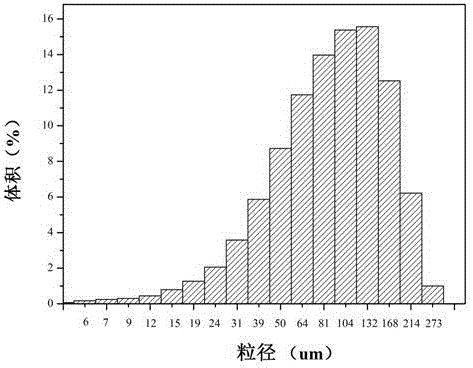
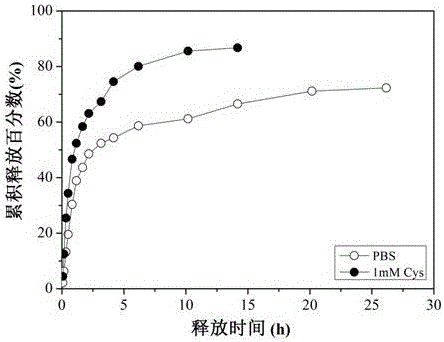
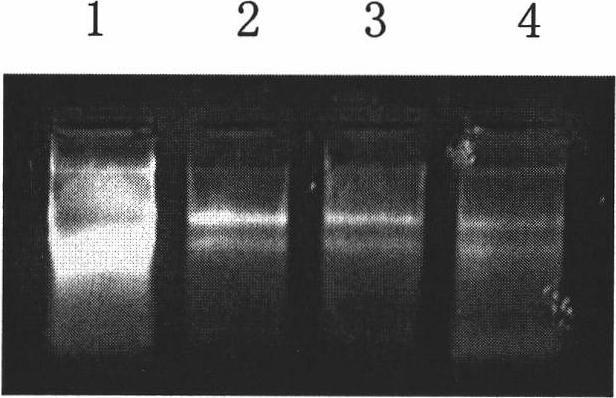
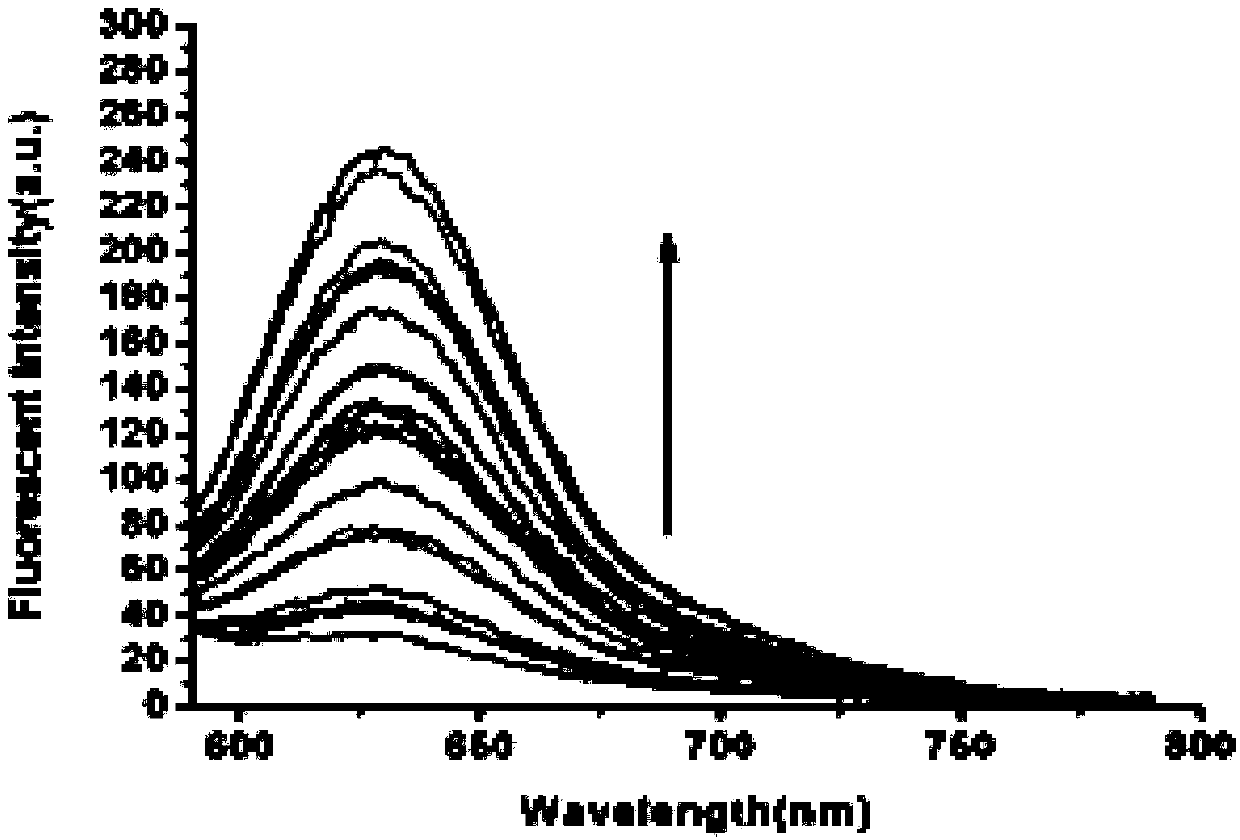
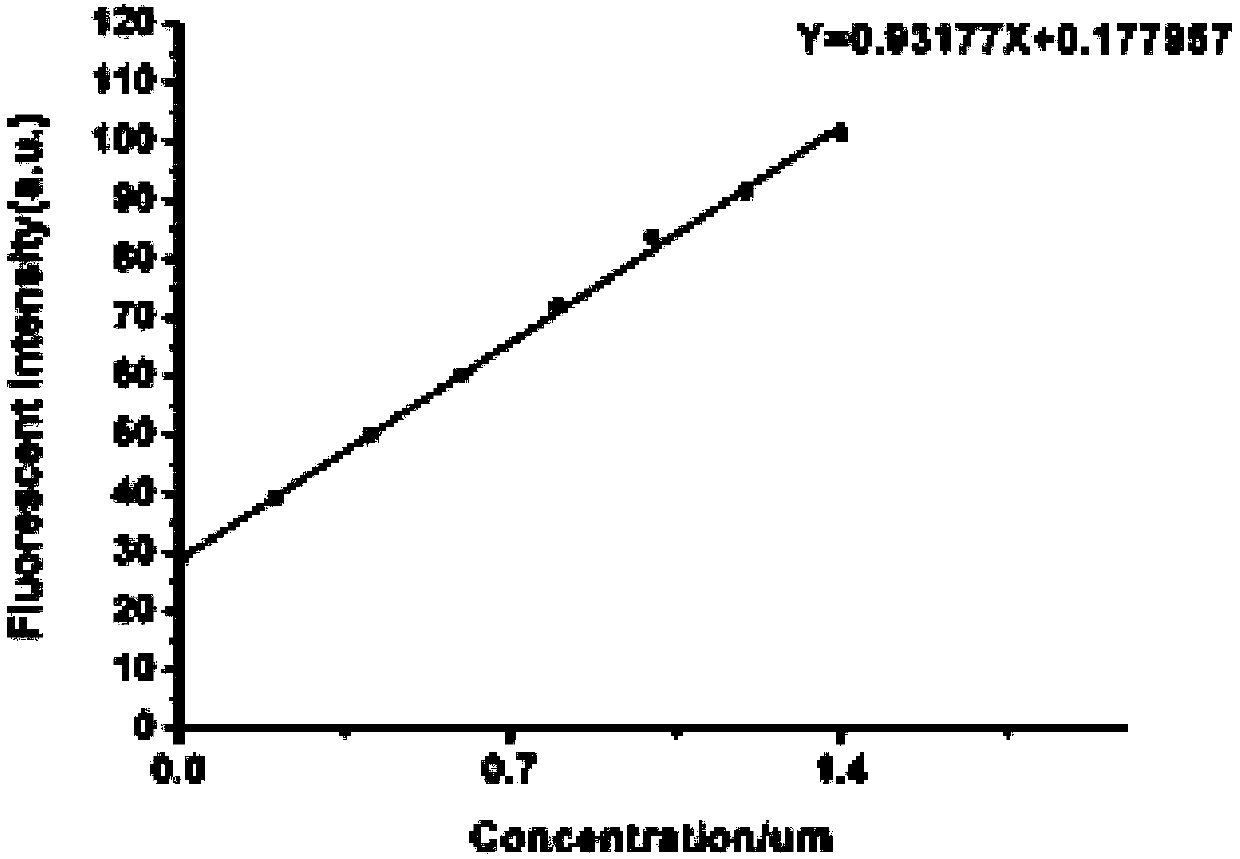
Dithiothreitol fluorescent probe as well as preparation method and application thereof
InactiveCN108003866AStrong specificityHigh sensitivityOrganic chemistryFluorescence/phosphorescenceThiolSpecific detection
The invention provides a fluorescent probe for detecting dithiothreitol in cells based on a xanthene structure. The probe is cheap and easily available, is simple in use, can realize specific recognition with the dithiothreitol to perform reaction so as to generate a xanthene fluorescent dye with higher fluorescence intensity, can not be disturbed by thiol and other reducing substances in the process of detecting the dithiothreitol, has excellent selectivity on the dithiothreitol, and can perform specific detection on the dithiothreitol in living cells.
Owner:UNIV OF JINAN
Stable ischemia modified albumin testing kit
ActiveCN102147416AImprove stabilityReduce the binding forceColor/spectral properties measurementsBiological testingRoom temperatureOxygen
The invention discloses a stable ischemia modified albumin testing kit which comprises a cobalt ion reagent and a dithiothreitol (DTT) reagent. A small amount of nonionic surfactant is added in the dithiothreitol reagent, thus the combination of DTT in the reagent and free oxygen is greatly reduced, the oxidation speed of DTT is reduced, and the stability of the reagent is remarkably improved. Simultaneously, the concentration of DTT is increased, thus the stability of the kit is improved to a certain extent. The stable ischemia modified albumin testing kit disclosed by the invention can be stored stably in an opened bottle in a dark place at the room temperature for 30 days, and also can be stored stably in a closed bottle at the temperature of 2 to 8 DEG C for 12 months, and can totally meet the requirements for clinical examinations.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com