Swab eluting solution with sample preservation and inactivation functions
An eluent and inactivation technology, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA preparation, etc., can solve the problems of no preservation effect of RNA samples, hazards to operators, and high cost, so as to reduce Exposure to risk of infection, maintenance of stability, effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Preparation of swab eluate:
[0022] Take a beaker of appropriate volume, weigh the reagents according to the formula in Table 1 below, add 400 ml of deionized water to the beaker, heat and stir to dissolve, after returning to room temperature, adjust the pH to 6.0 with hydrochloric acid, make up to 1L of deionized water, and after stirring, Prepare 1 L of swab eluate.
[0023] Table 1
[0024]
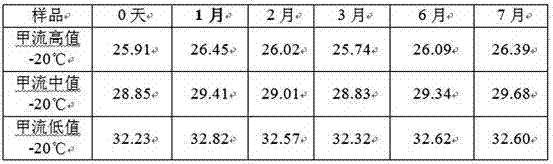
[0025] 2. Specimen collection and processing: Take swab specimens from patients who were identified as positive for influenza A by clinical PCR. The virus titer was representative, and the virus titers were high, medium and low (the CT value detected by Realtime PCR was about 26, 29, and 32, respectively). 8ml swab eluate in a 10ml centrifuge tube;
[0026] Vortex and shake on a vortexer for 5s, discard the swab, and dispense the eluate in 2ml / piece;
[0027] The eluate was placed at four temperatures of 37°C, normal temperature, 4°C, and -20°C for thermal stability as...
Embodiment 2
[0060] 1. Preparation of swab eluate
[0061] Take a beaker of appropriate volume, weigh the reagents according to the formula in Table 7 below, add 400ml of deionized water to the beaker, heat and stir to dissolve, after returning to room temperature, adjust the pH to 6.0 with hydrochloric acid, make up to 1L of deionized water, and after stirring, That is, 1 L of swab eluate is prepared.
[0062] Table 7
[0063]
[0064] 2. Specimen collection and processing: Take swab samples from patients with positive mycoplasma identified by clinical PCR. The virus titer is representative, and the virus titer is high, medium and low (the CT value of the sample is about 23, 26, and 30, respectively), and immersed in 8ml of Swab eluate in a 10ml centrifuge tube;
[0065] Vortex and shake on a vortexer for 5s, discard the swab, and dispense 2ml / piece;
[0066] The eluates were placed at 37°C, normal temperature, 4°C, and -20°C for stability assessment.
[0067] The eluate was placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com