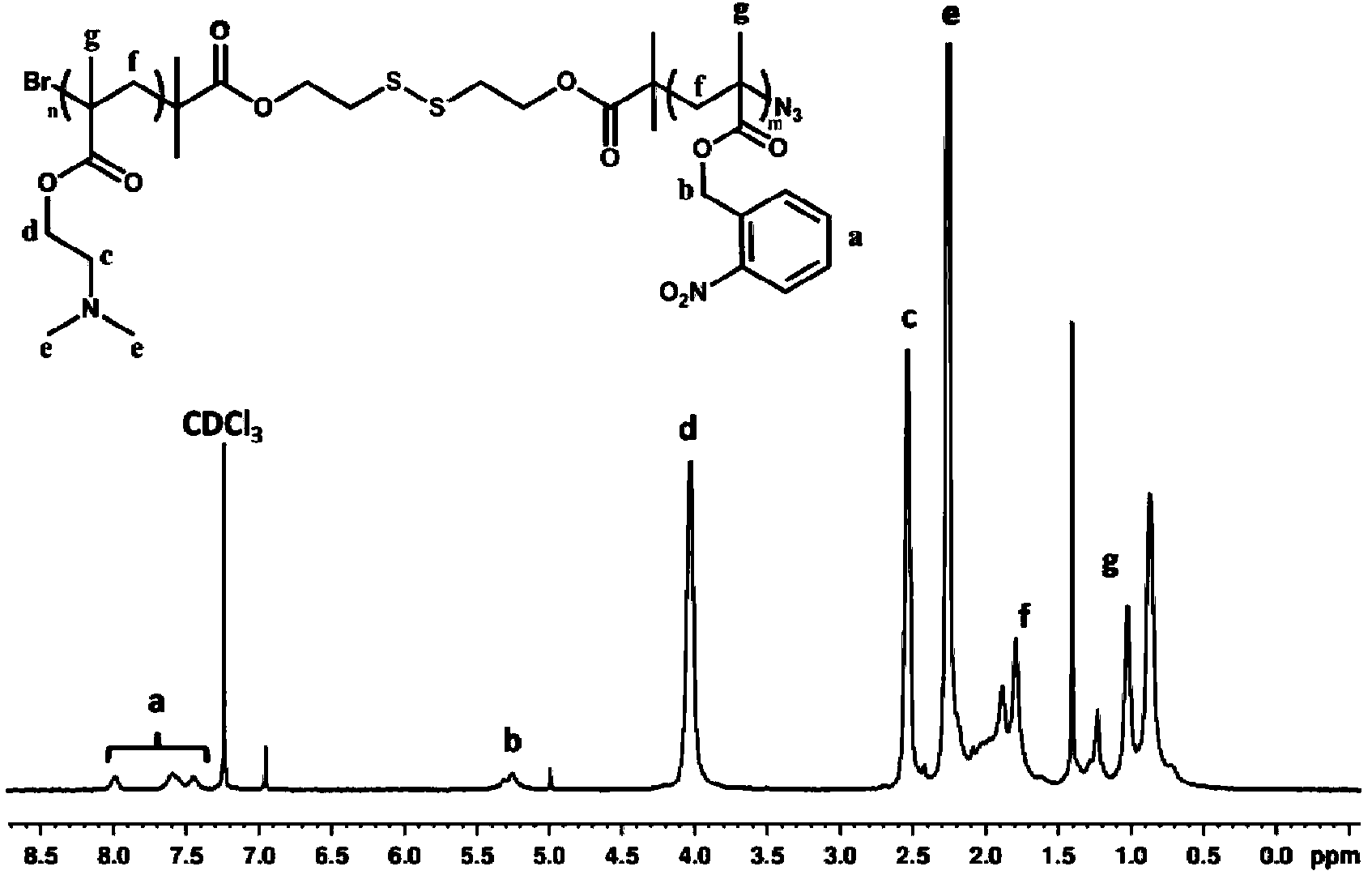
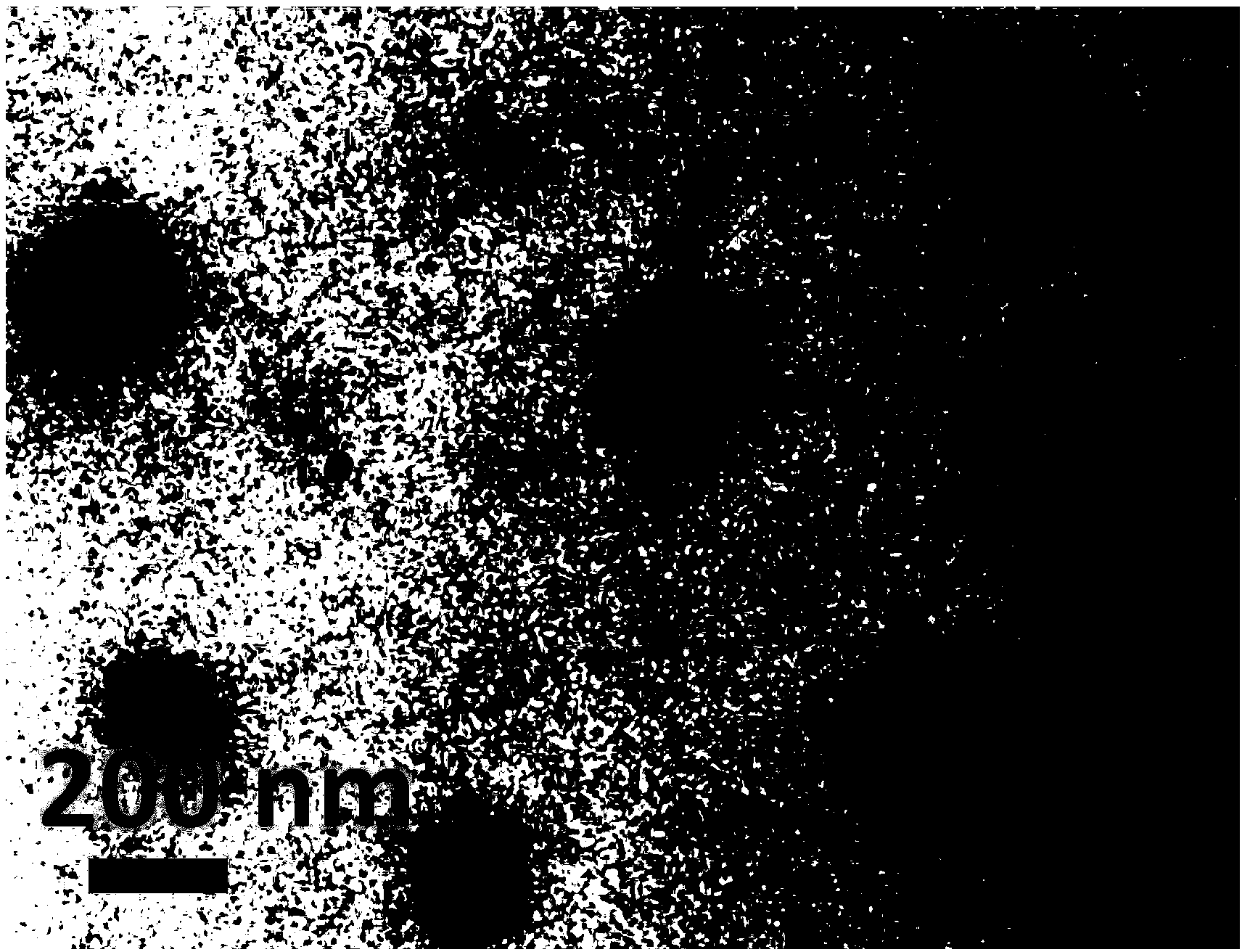Preparation method and application of quadruple-responsiveness block micelle
A responsive, micellar technology, which is applied in the field of preparation of quadruple responsive block micelles, can solve the problem that the precise regulation of polymer substances does not achieve the desired effect, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] Step 1: Preparation of 2-hydroxy-2'-(bromoisobutyryl)ethyl disulfide: Mix 4.62 g of 2-hydroxyethyl disulfide, 4 mL of triethylamine, and 100 mL of dry THF to dry round bottom flask and placed in an ice water bath. 4. A solution of 598 g of 2-bromoisobutyryl bromide in 40 ml of tetrahydrofuran was gradually added dropwise to a round bottom flask, the entire reaction system was protected by nitrogen, and stirred at room temperature for 16 hours. After the reaction is over, remove triethylamine hydrochloride, spin off tetrahydrofuran, dissolve in dichloromethane, wash once with aqueous sodium hydroxide solution in a separatory funnel to make it alkaline, and then wash with deionized water until neutral. , to obtain the organic layer. The organic solution was dried overnight with anhydrous magnesium sulfate to obtain a crude product, and purified 2-hydroxy-2'-(bromoisobutyryl)ethyl disulfide was obtained by silica gel column chromatography.
[0042] Step 2: Preparation of...
example 2
[0048] Step 1: Preparation of 2-hydroxy-2'-(bromoisobutyryl)ethyl disulfide: Mix 2-hydroxyethyl disulfide, triethylamine, and dry THF into a dry round bottom flask, and Place in ice water bath. The tetrahydrofuran solution of 2-bromoisobutyryl bromide was gradually added dropwise to the round bottom flask, the whole reaction system was protected by nitrogen, and stirred at room temperature for 24 hours. After the reaction is over, remove triethylamine hydrochloride, spin off tetrahydrofuran, dissolve in dichloromethane, wash once with aqueous sodium hydroxide solution in a separatory funnel to make it alkaline, and then wash with deionized water until neutral. , to obtain the organic layer. The organic solution was dried overnight with anhydrous magnesium sulfate to obtain a crude product, which was subjected to silica gel column chromatography with an eluent of diethyl ether and n-hexane (3 / 7 V / V) to obtain pure 2-hydroxy-2'-(bromoisobutyl acyl) ethyl disulfide. Wherein th...
Embodiment 3
[0055] Step 1: Preparation of 2-hydroxy-2'-(bromoisobutyryl)ethyl disulfide: Mix 2-hydroxyethyl disulfide, triethylamine, and dry THF into a dry round bottom flask, and Place in ice water bath. The tetrahydrofuran solution of 2-bromoisobutyryl bromide was gradually added dropwise to the round bottom flask, the whole reaction system was protected by nitrogen, and stirred at room temperature for 24 hours. After the reaction is over, remove triethylamine hydrochloride, spin off tetrahydrofuran, dissolve in dichloromethane, wash once with aqueous sodium hydroxide solution in a separatory funnel to make it alkaline, and then wash with deionized water until neutral. , to obtain the organic layer. The organic solution was dried overnight with anhydrous magnesium sulfate to obtain a crude product, which was subjected to silica gel column chromatography with an eluent of diethyl ether and n-hexane (3 / 7 V / V) to obtain pure 2-hydroxy-2'-(bromoisobutyl acyl) ethyl disulfide. Wherein th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com