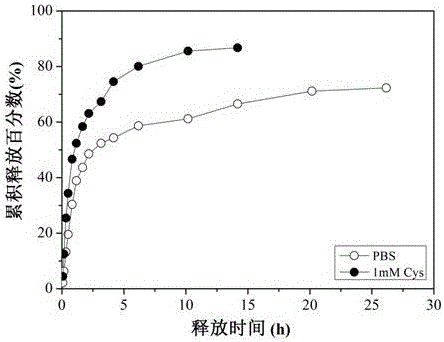
Enzymatic catalysis crosslinking reduction-responsive hyaluronic acid microgel and preparation method thereof
A technology of hyaluronic acid and microgel, which is applied in the directions of non-active ingredients, such as medical preparations, pharmaceutical formulations, fermentation, etc., can solve the problems of application limitation, reduced activity, and the three-dimensional network structure does not have reduction responsiveness, and achieves solidification. The effect of fast speed, overcoming toxic side effects, excellent biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of thiolated hyaluronic acid macromolecule (HA-SH).
[0026] Prepare 100 mL of sodium hyaluronate aqueous solution with a concentration of 2 wt%, add such N -Hydroxysuccinimide 2.3g and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC .HCL) 3.832 g into the sodium hyaluronate solution, adjust the pH of the solution to about 5.4, stir at room temperature for 1 h, add 3.372 g of cystamine dihydrochloride, stir at room temperature for 48 h, and dialyze the reaction solution with distilled water for 24 h to remove ungrafted Cystamine dihydrochloride. Add 4.6275 g of DL-dithiothreitol (DTT) to the dialysate, adjust the pH of the solution to 8.5, and react for 24 hours in a dark nitrogen atmosphere. After the reaction, adjust the pH of the reaction solution to 5, and dialyze with twice distilled water for 3 days , freeze-dried to obtain a white flocculent product, and the free sulfhydryl content was 6.5% as measured by Ellman's reagent.
Embodiment 2
[0028] Preparation of hyaluronic acid microgels by cross-linking catalyzed by horseradish peroxidase in inverse emulsion method.
[0029] Preparation of the aqueous phase: Weigh 150 mg of the mercaptolated hyaluronic acid synthesized in Example 1, prepare a 0.4% (w / v) aqueous solution with double distilled water, add tyramine hydrochloride to the aqueous solution, and its final concentration to 50 mM, the aqueous solution was adjusted to pH=7 with dilute NaOH solution. Preparation of oil phase: take liquid paraffin as the oil phase according to the water phase / oil phase volume ratio of 1 / 4, and add Span80 / Tween80=88 / 12 (v / v) mixed surface activity at a concentration of 1 wt%. agent. The water phase was slowly added to the oil phase, emulsified at 1000 rpm for 30 min at high speed, and after forming stable colostrum, horseradish peroxidase (1000 units / mL, 0.48 mL) was added, and stirred at 600 rpm for 4 h to solidify the microspheres. After the reaction was completed, they we...
Embodiment 3
[0031] Surface morphology analysis of hyaluronic acid microgels.
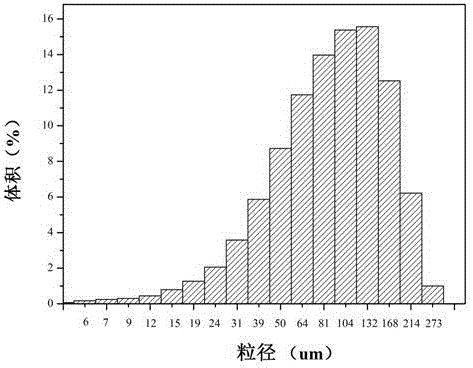
[0032] Take an appropriate amount of freeze-dried microgel and disperse it in double-distilled water, and dehydrate it with 20%, 40%, 60%, 80%, 90%, and 100% absolute ethanol, respectively. Use a dropper to take an appropriate amount of ethanol suspension and drop it on a clean silicon wafer, dry it naturally, and use a scanning electron microscope for morphology analysis after spraying gold, as shown in figure 1 As shown, the particle size of the dried microgel is about 10 um.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com