Dithiothreitol fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probe and synthesis method, which is applied in the field of analytical chemistry, can solve the problems that fluorescent probes do not have rapid response sensitivity, etc., and achieve the effects of easy promotion, high sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
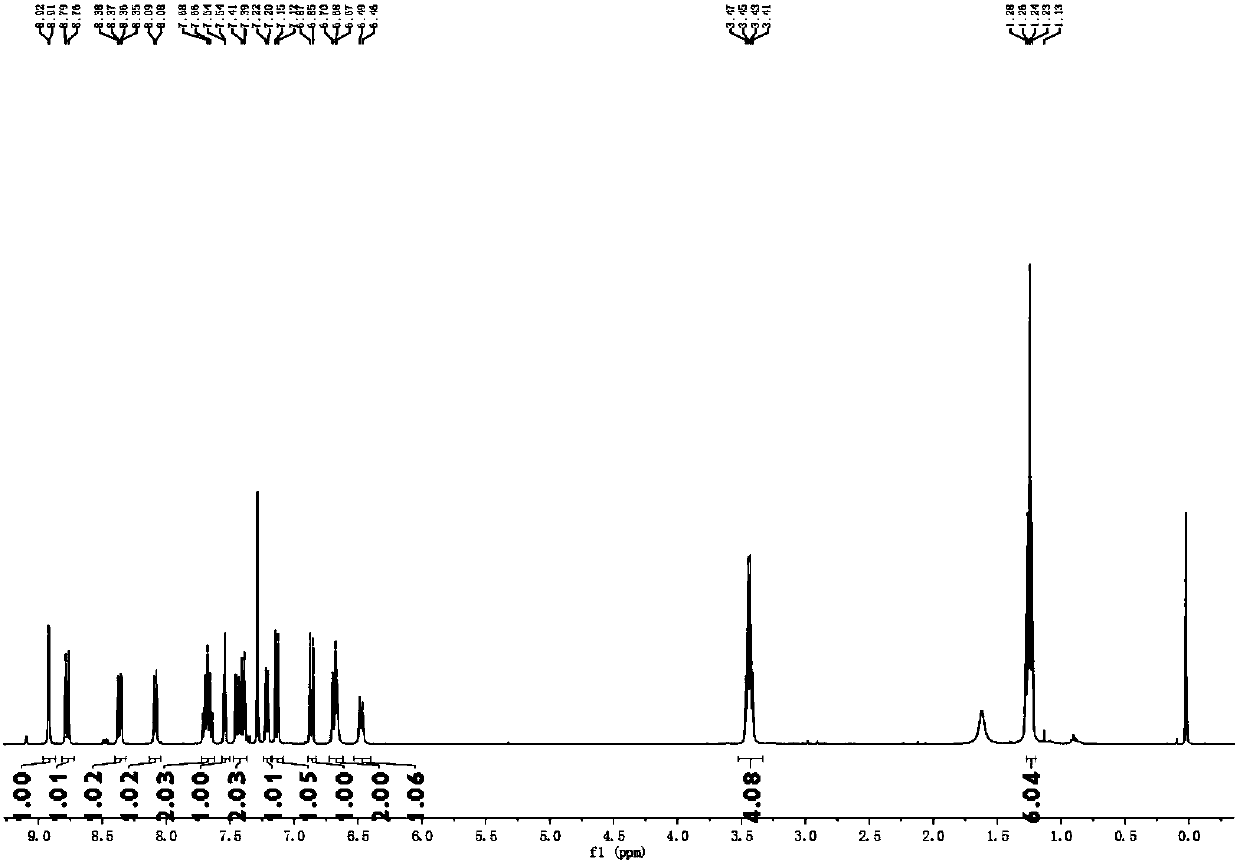
[0032] Example 1 Preparation of Fluorescent Probes
[0033] (1) 4-diethylamino keto acid and 1,6-naphthalenediol use concentrated sulfuric acid as solvent, reflux at 90°C; then cool the reaction solution to -5-0°C, add perchloric acid to make it precipitate, Suction filtration, drying and column purification, eluent dichloromethane:methanol=10:1 for purification, to obtain compound 3:
[0034] ;
[0035] (2) Add compound 3 (1 mmol) obtained in step 1, 2,4-dinitrofluorobenzene (1 mmol) and N,N-dimethylformamide (5 mL) into a 50 mL one-necked flask, 50 °C for 5 hours. Cool to room temperature, extract with dichloromethane (15mL) and purified water (15mL) for 3 to 5 times, collect the organic layer, dry with anhydrous magnesium sulfate and spin dry, and dichloromethane:methanol=50:1 for the obtained solid The eluent is purified by column chromatography, and the pink product is obtained after removing the solvent, which is the fluorescent probe for detecting intracellular DTT...
Embodiment 2
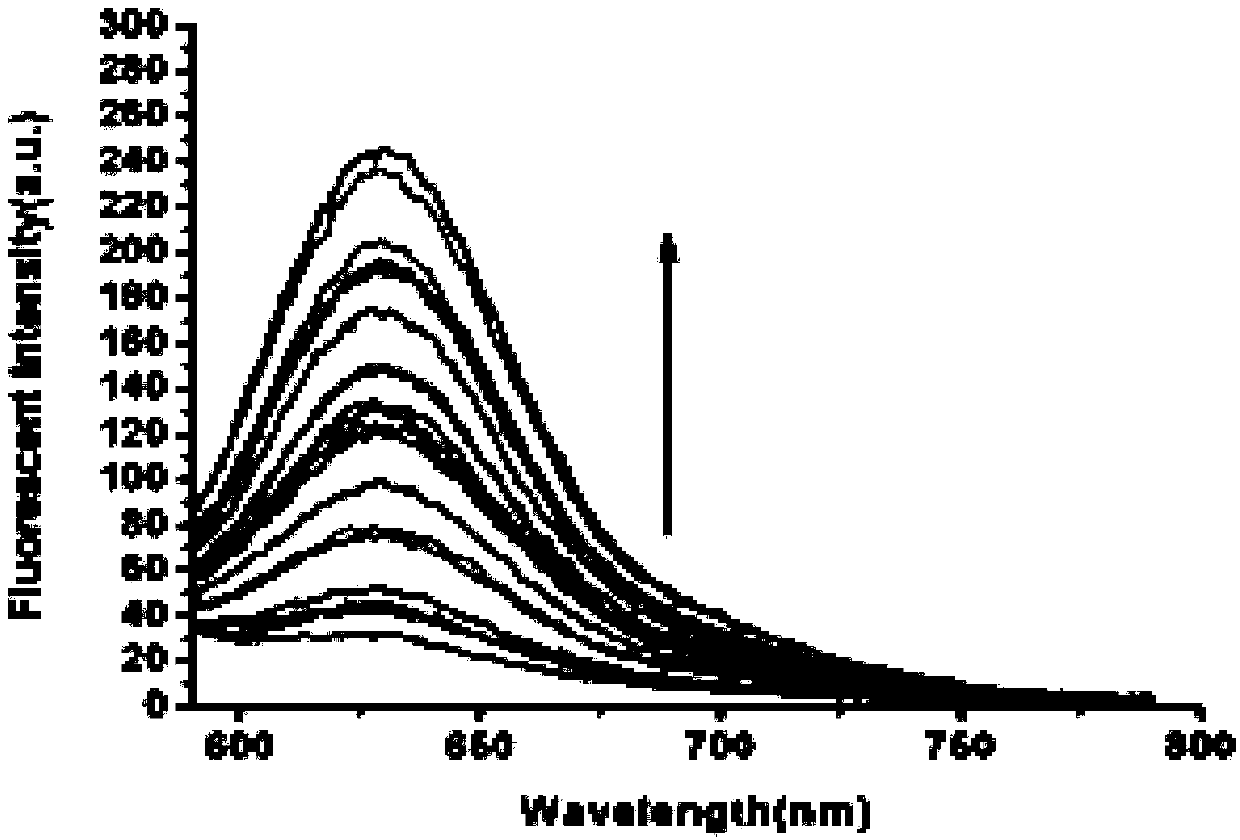
[0036] Example 2 Fluorescence spectra of fluorescent probes at different DTT concentrations
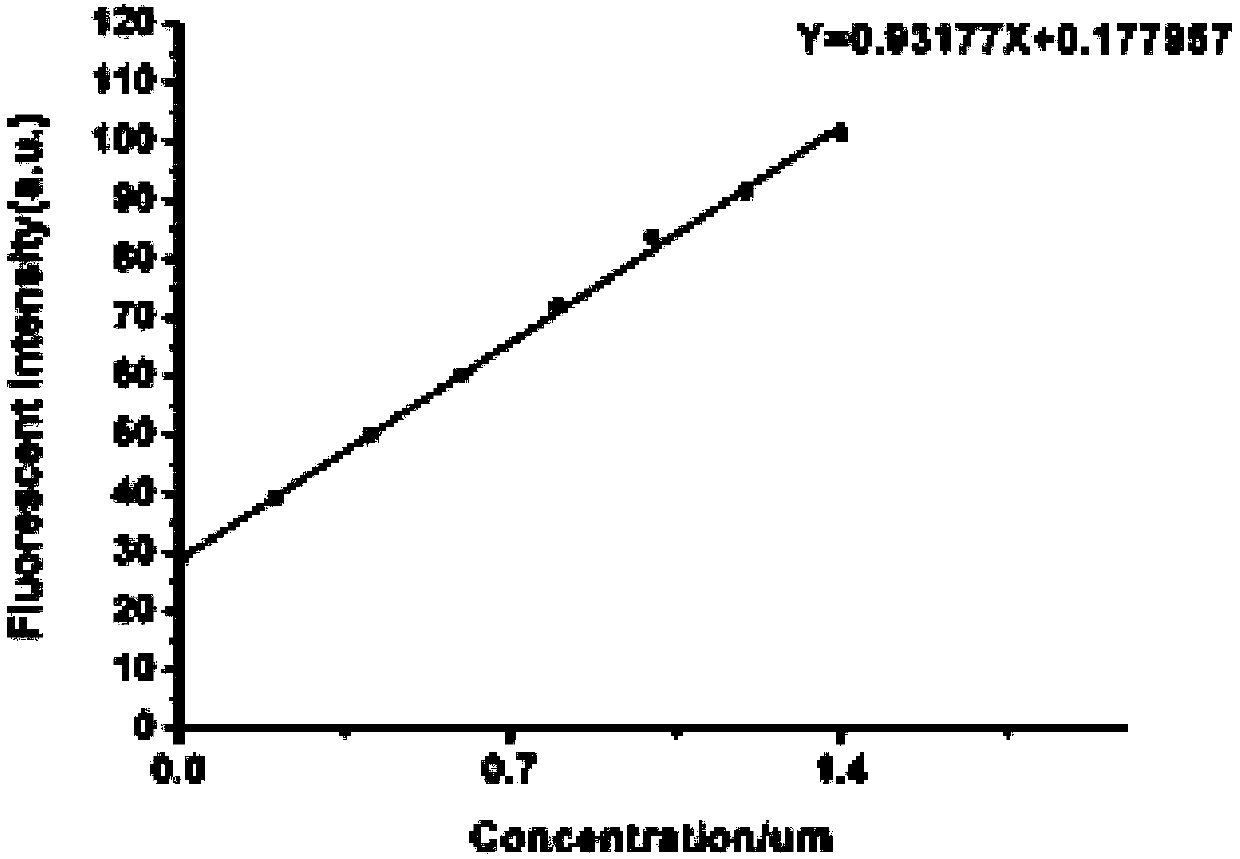
[0037] Prepare in advance 18 parts of 5 mL phosphate-buffered saline (PBS) containing 10 μM fluorescent probe in Example 1, containing 20% acetonitrile, and the DTT concentrations are 0, 2, 4, 6, 8, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 μM. Fluorescence detection is then performed (λ ex = 580nm); Calculate the fluorescence intensity in each system; Evaluate the response performance of the probe to DTT, such as figure 2 shown. Depend on figure 2 It can be seen that within a certain concentration range, the fluorescence intensity is positively correlated with dithiothreitol, and the concentration of dithiothreitol can be determined by detecting different fluorescence intensities. Analyze the linear relationship between the fluorescence intensity at 638nm and the concentration of DTT, such as image 3 As shown, the regression equation is y=0.93177x+0.177957, where ...
Embodiment 3
[0038] Example 3 Fluorescence Intensities of Fluorescent Probes Reacted with Different Substances
[0039] Prepare 10 parts of 5mL buffer solution (containing 20% acetonitrile, pH = 7.4) containing 10 μM fluorescent probe in Example 1 in advance, and then add 100 μL of CuSO with a concentration of 100 μM to the system respectively 4 , MgSO 4 , CaCl 2 、CoCl 2 、NaNO 2 、Na 2 SO 3 、Na 2 SO 4 , ZnSO 4 , peroxy tert-butyl, peroxy tert-butanol, HClO, H 2 o 2 , NO, Na 2 S, SnSO 4 , KI, HgCl 2 , VC, Hcys, Cys, GSH, dithiothreitol in PBS solution. Fluorescence detection is then performed (λ ex = 580 nm); calculate the fluorescence intensity in each system, and evaluate the interference of the different substances on the fluorescent probe solution, such as Figure 4 shown. Depend on Figure 4 It can be seen that the dithiothreitol fluorescent probe can resist the influence of various interfering substances and has strong specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com