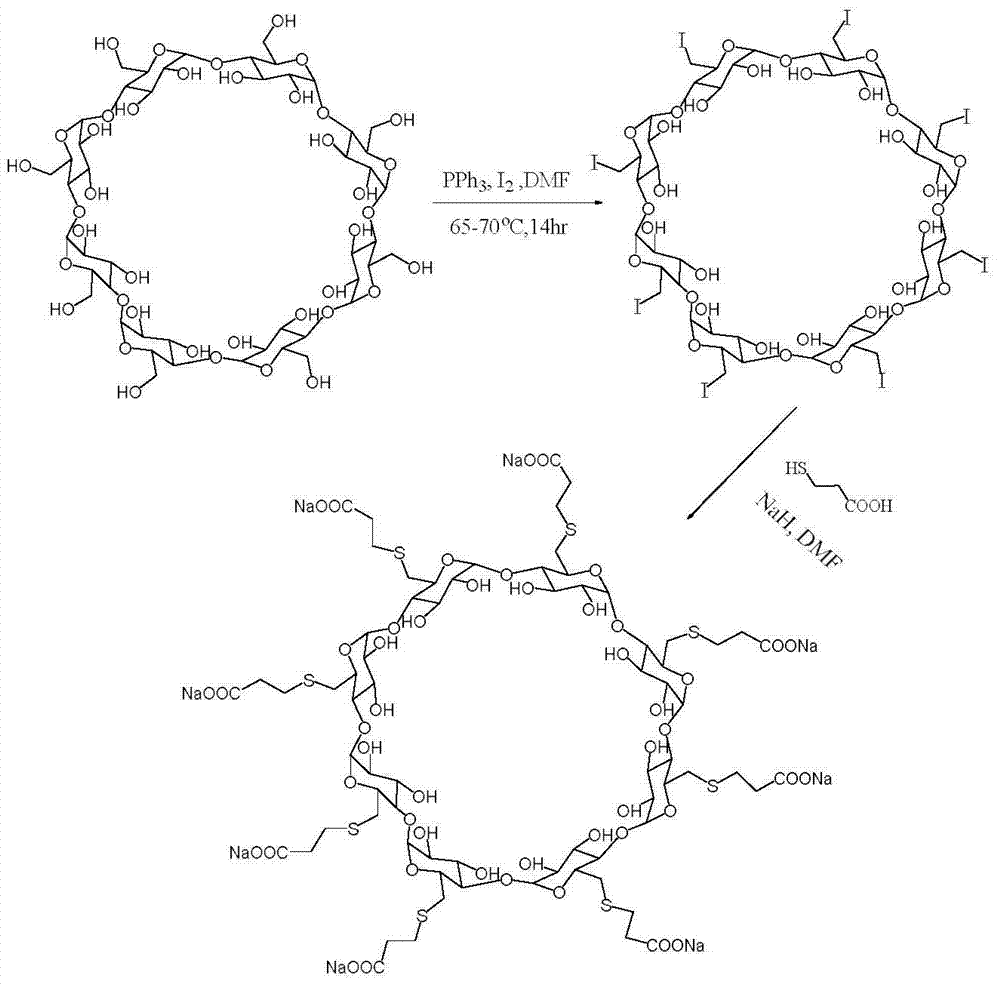
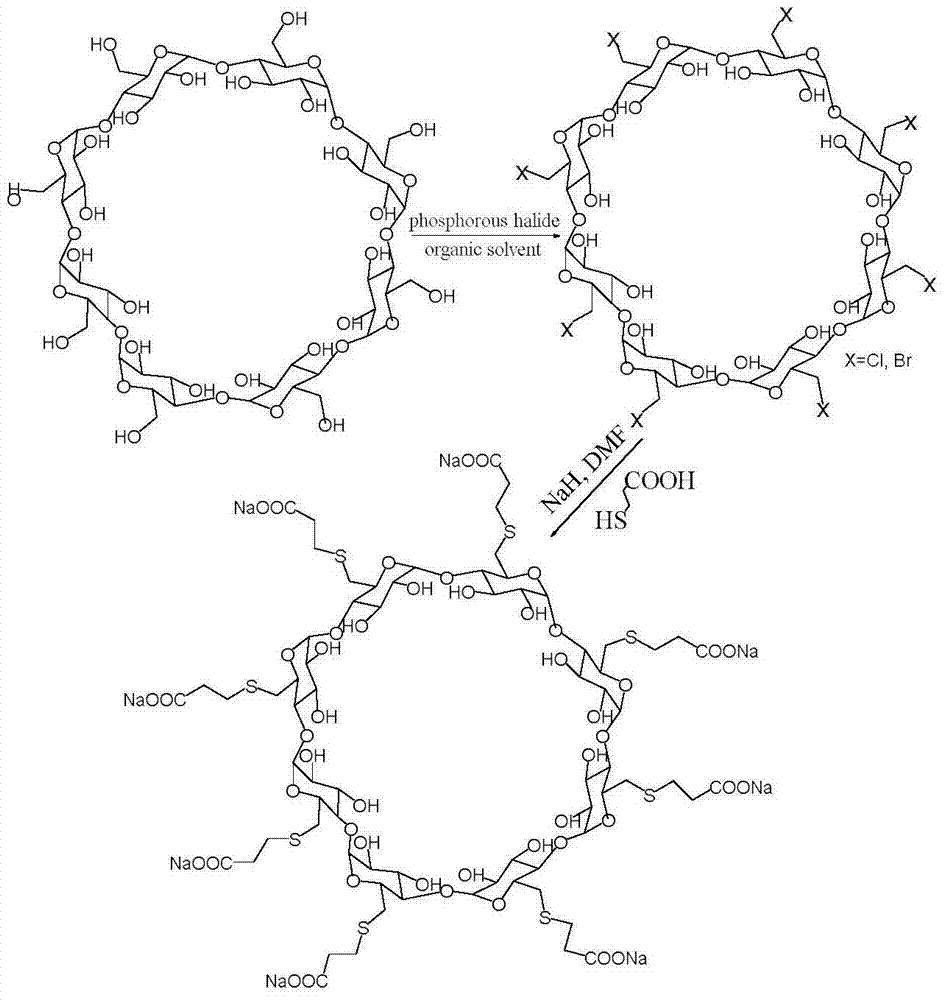
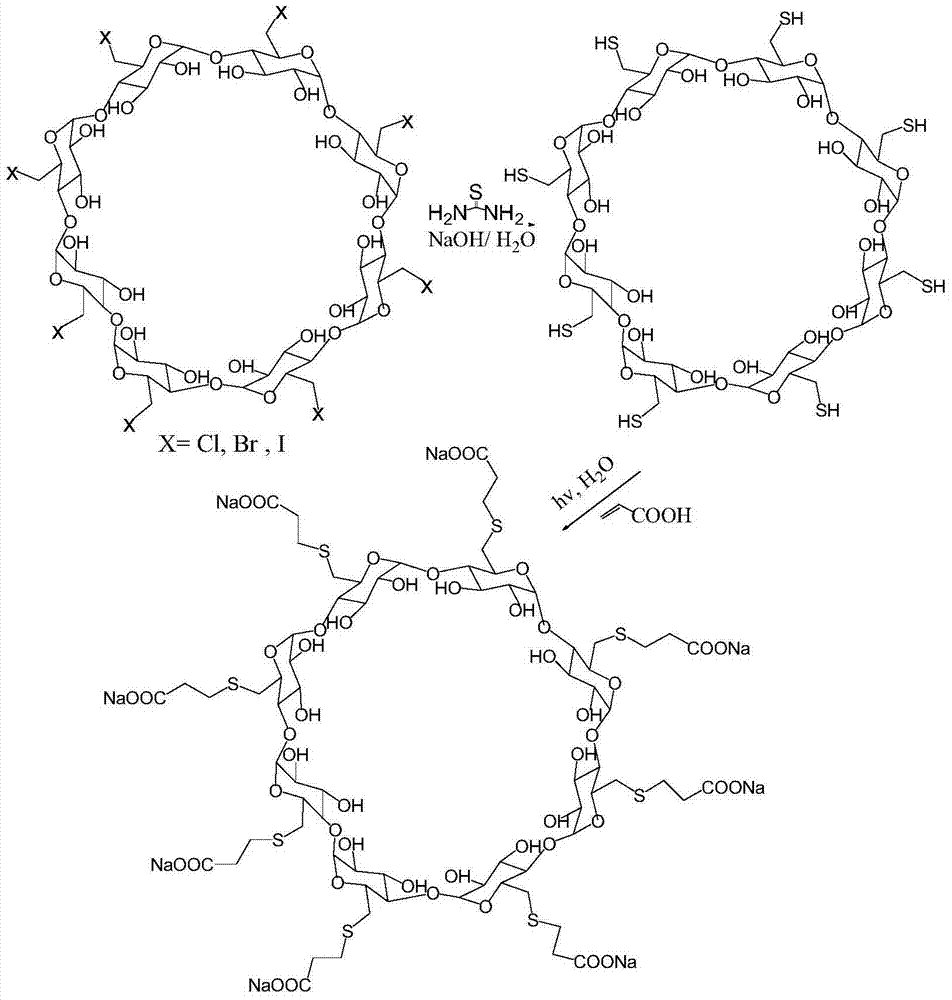
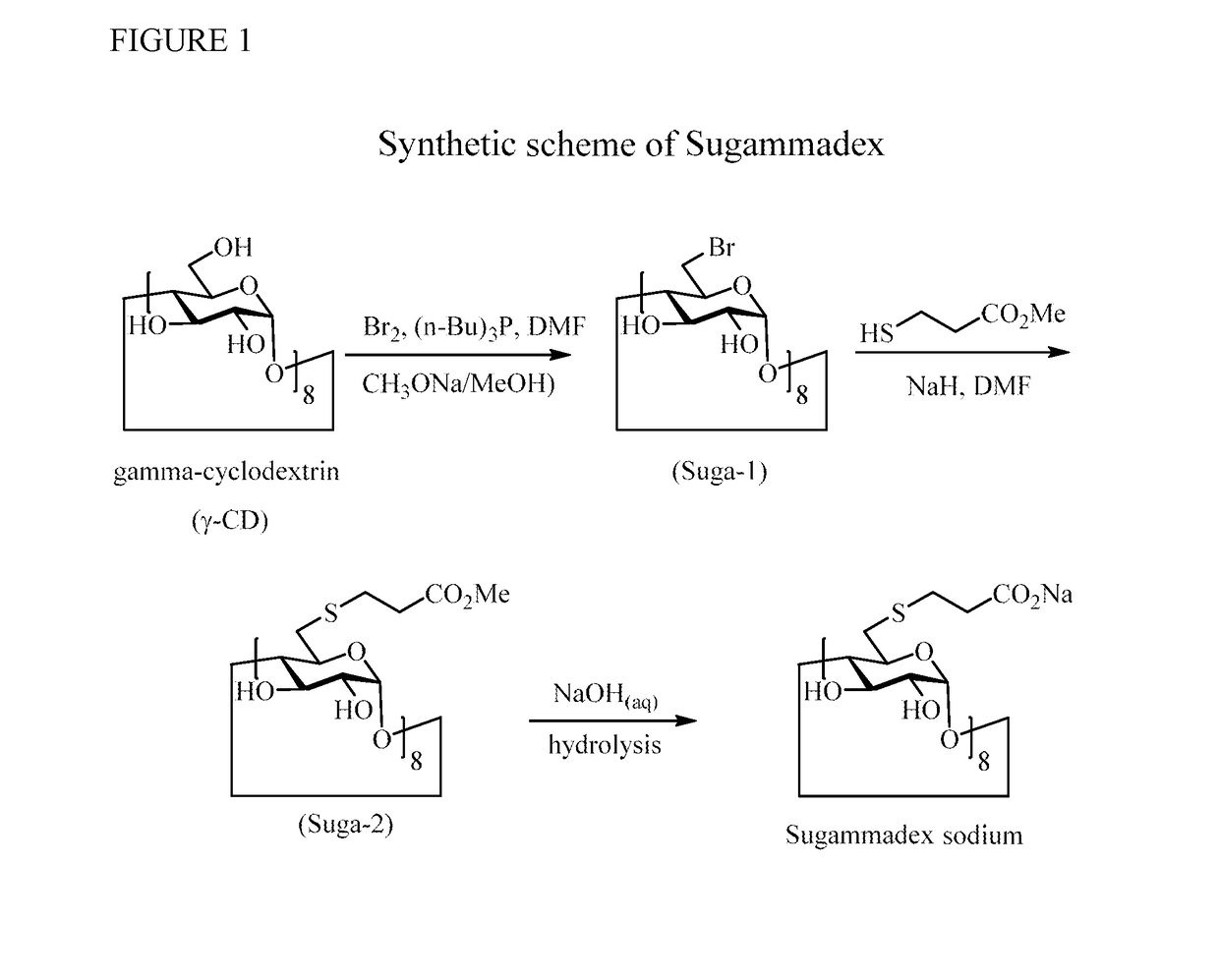
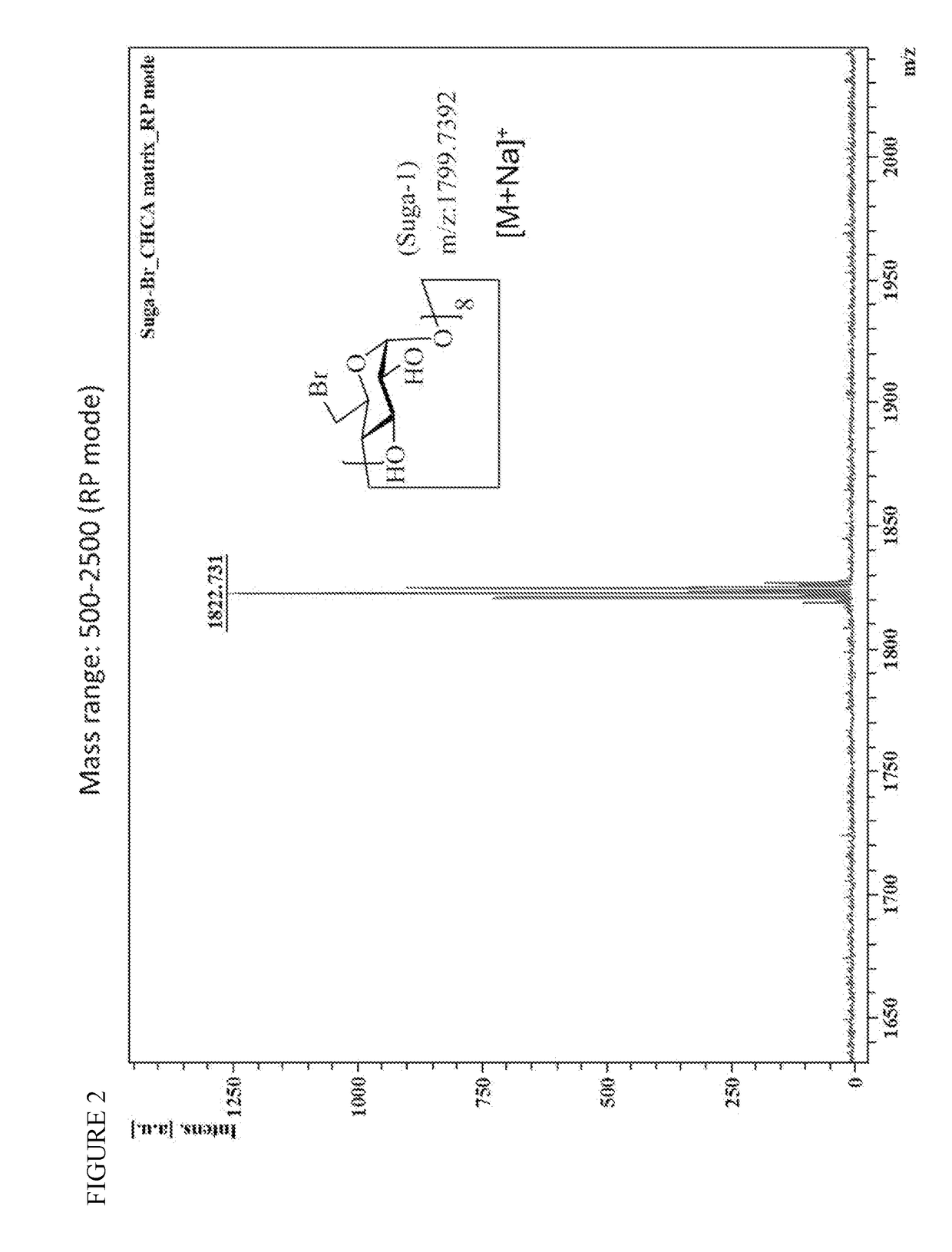
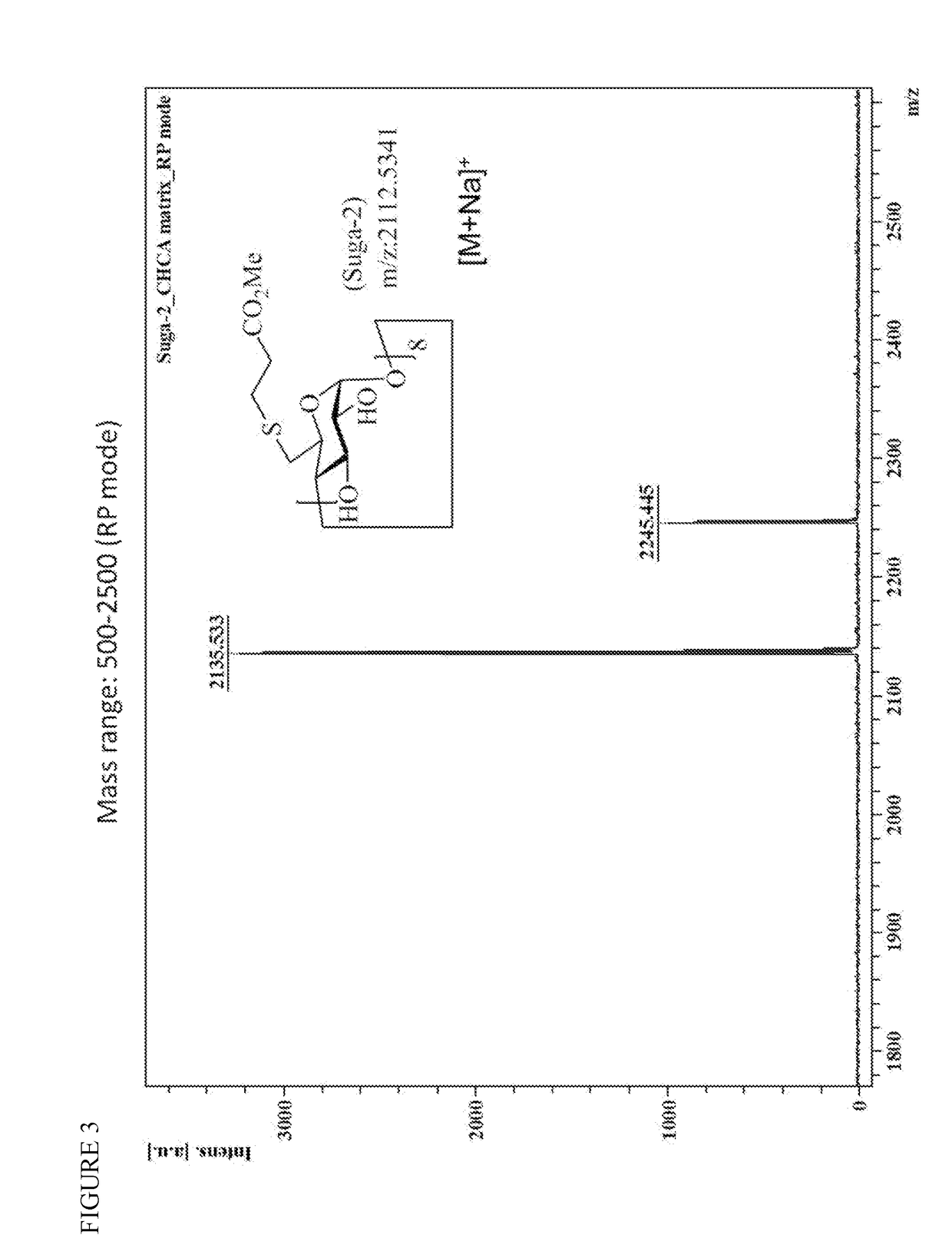
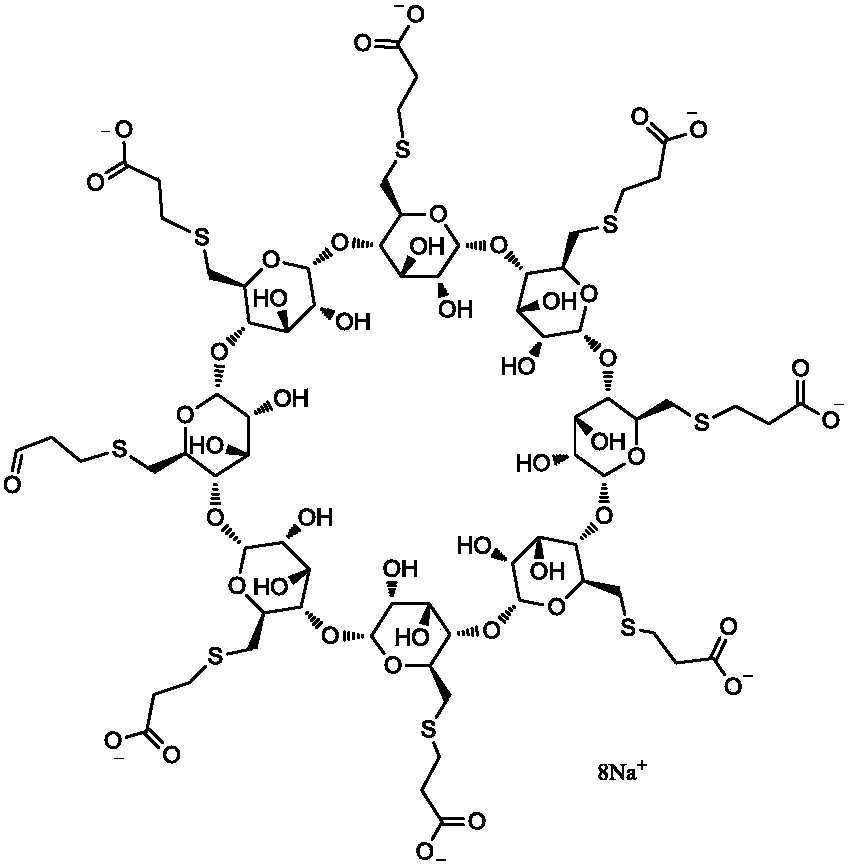
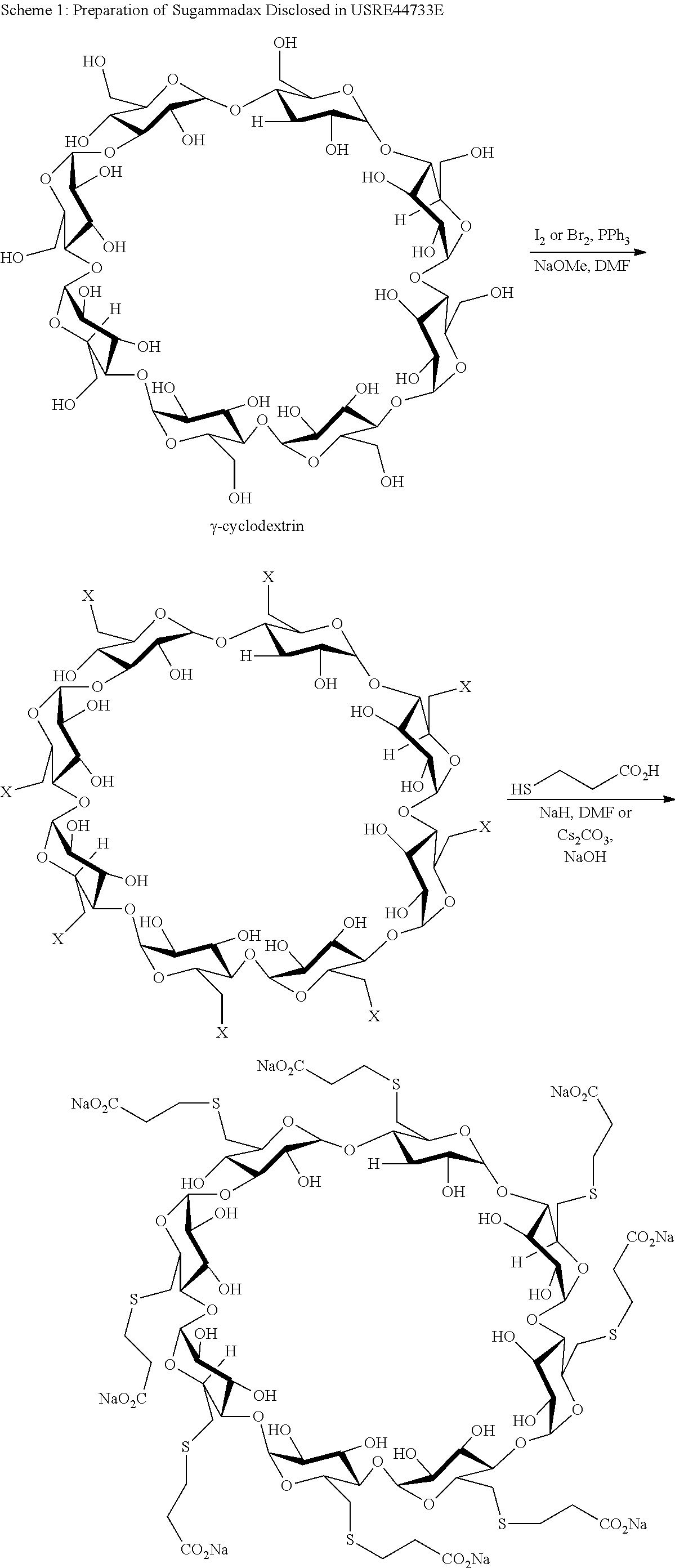
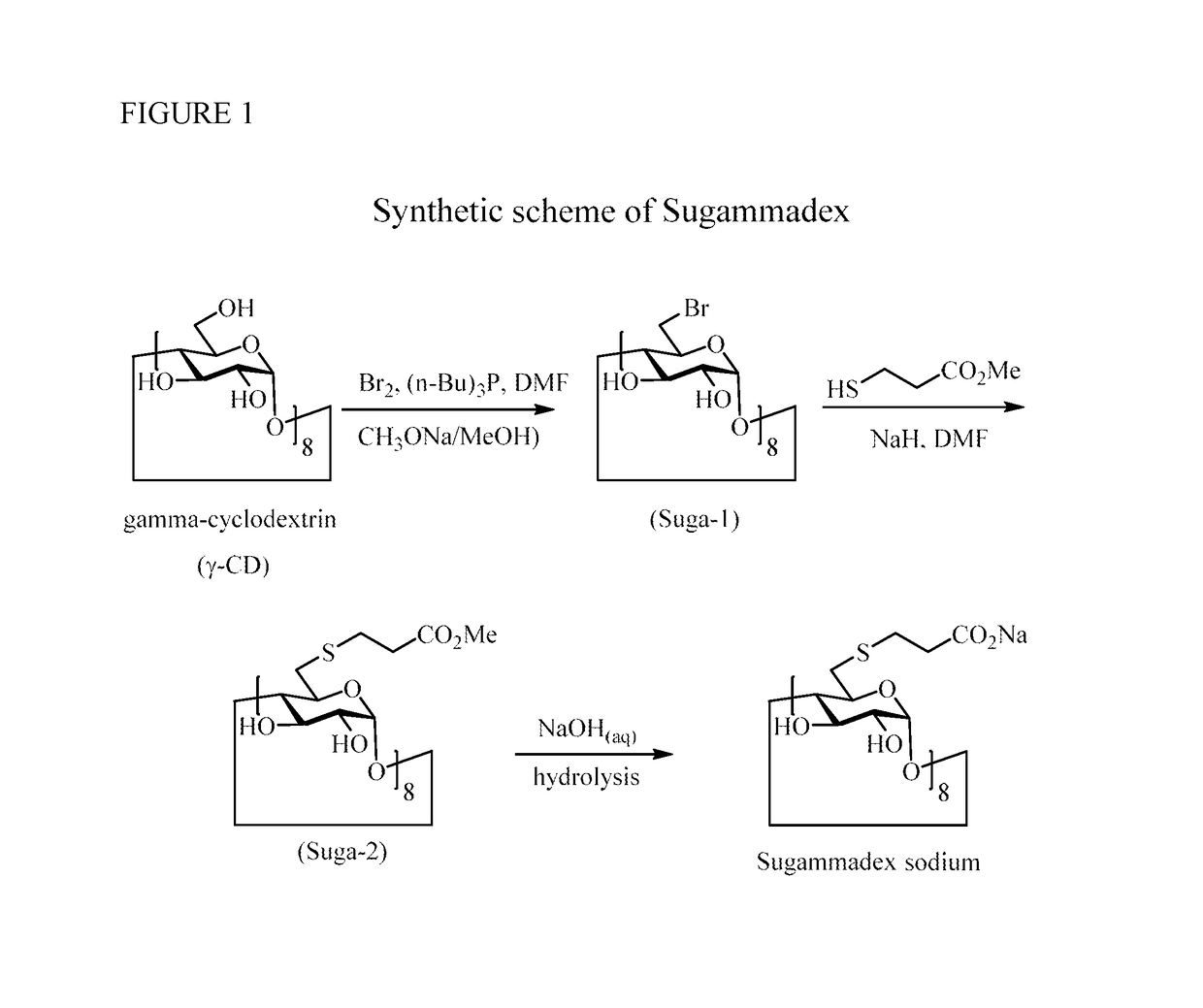
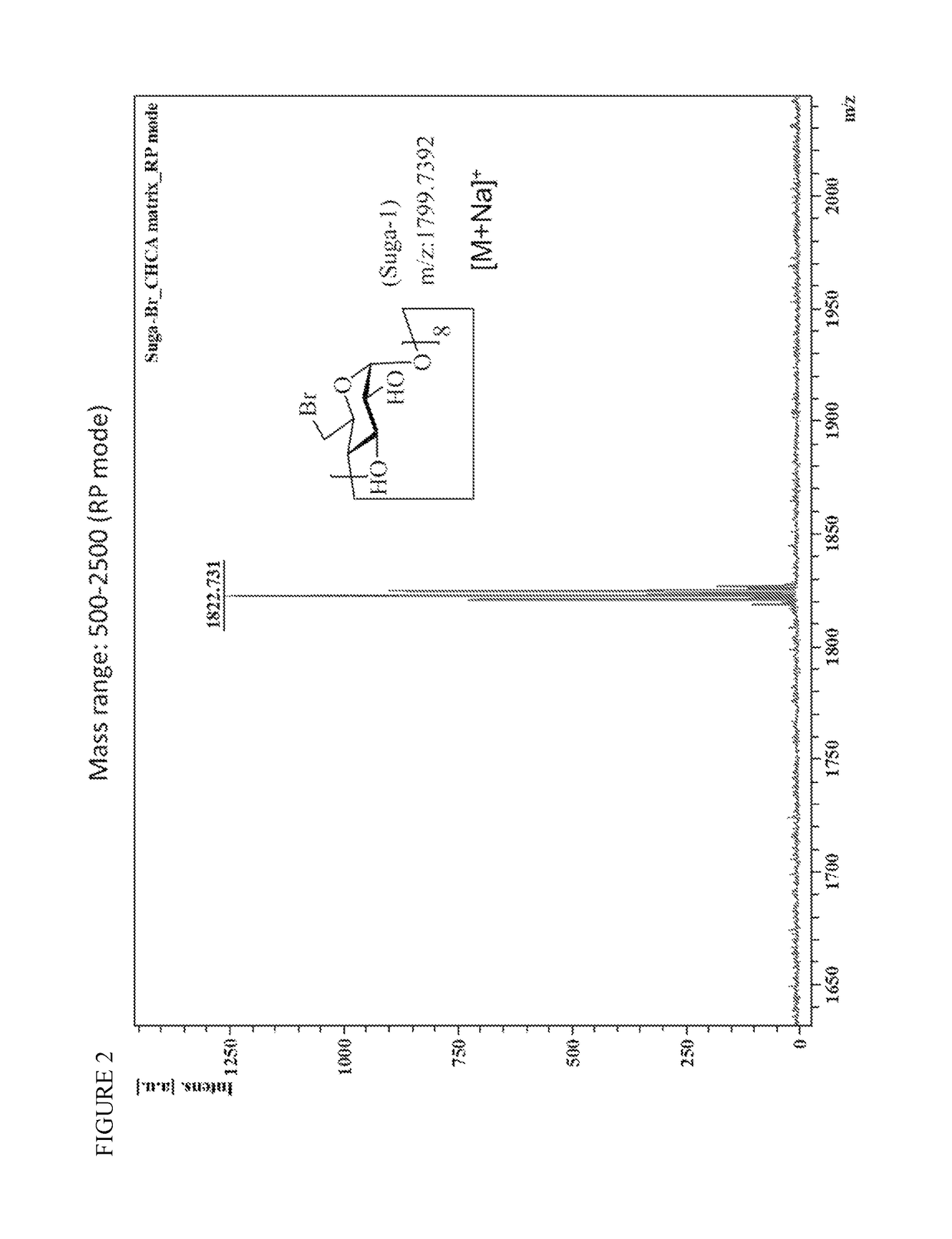
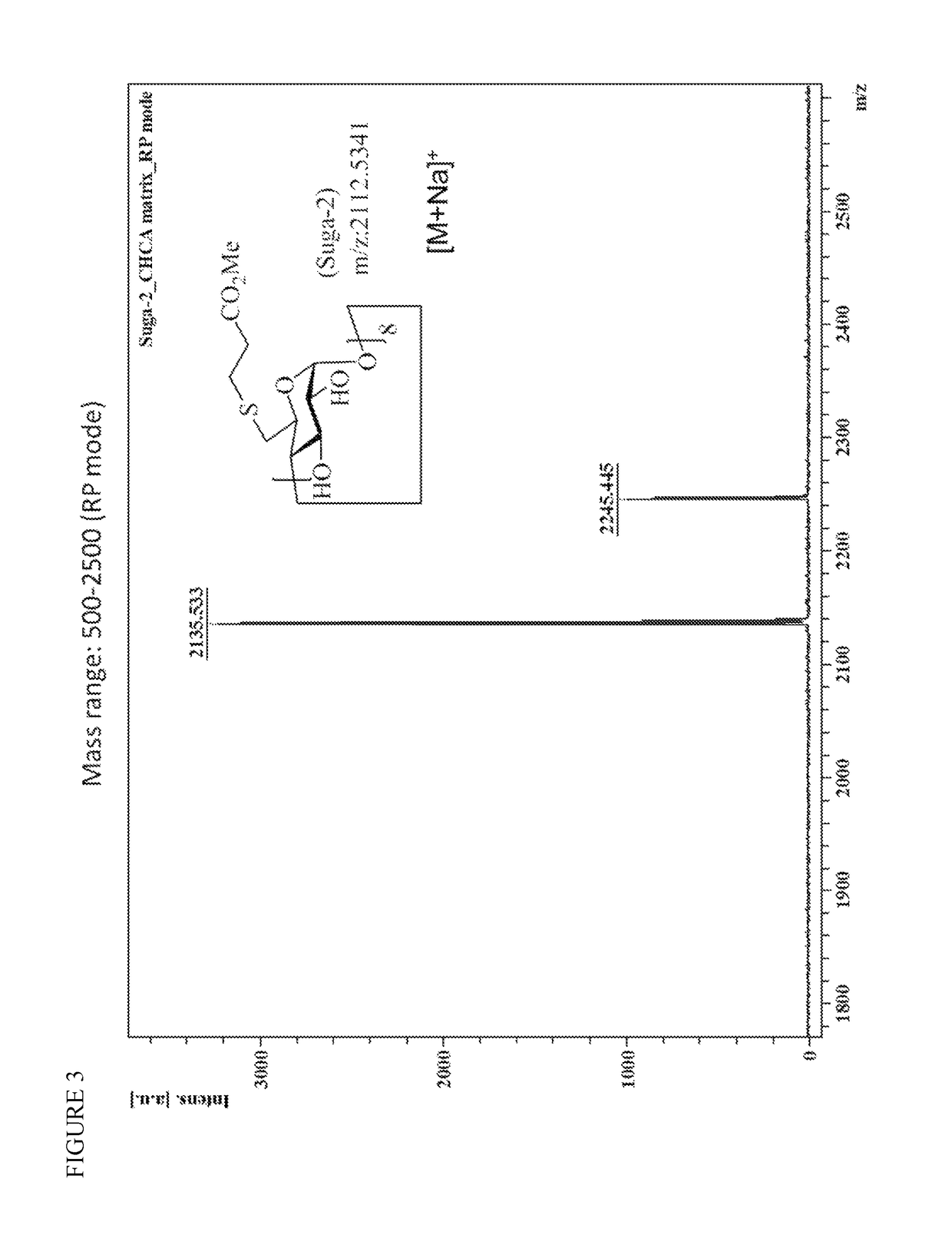
Patents
Literature
81 results about "Sugammadex Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
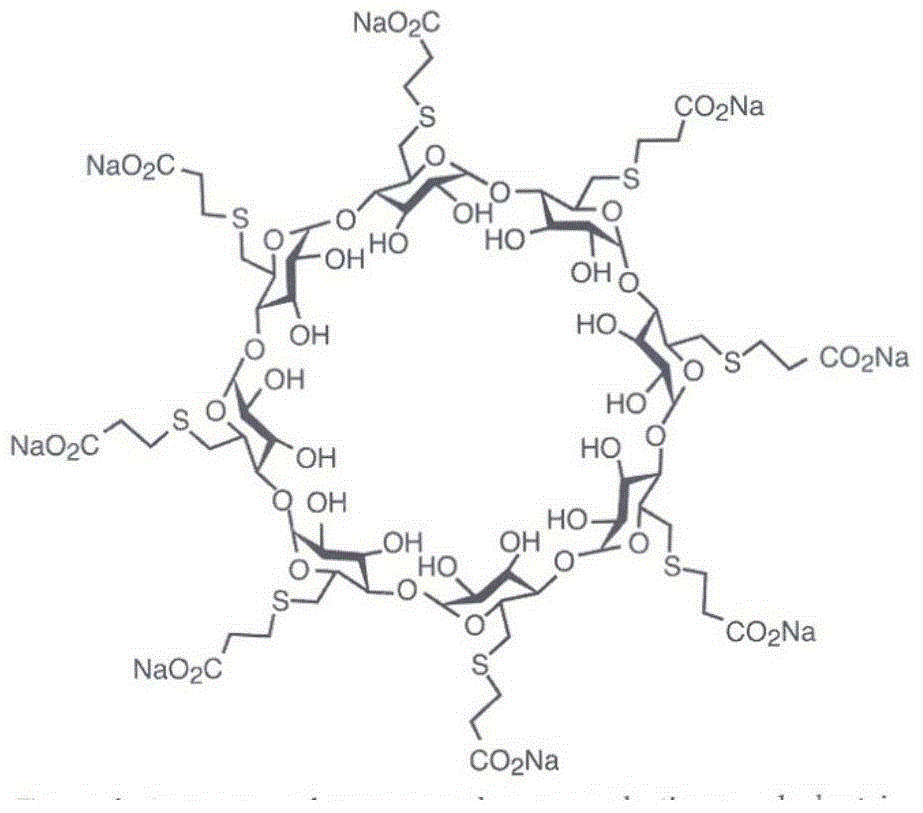
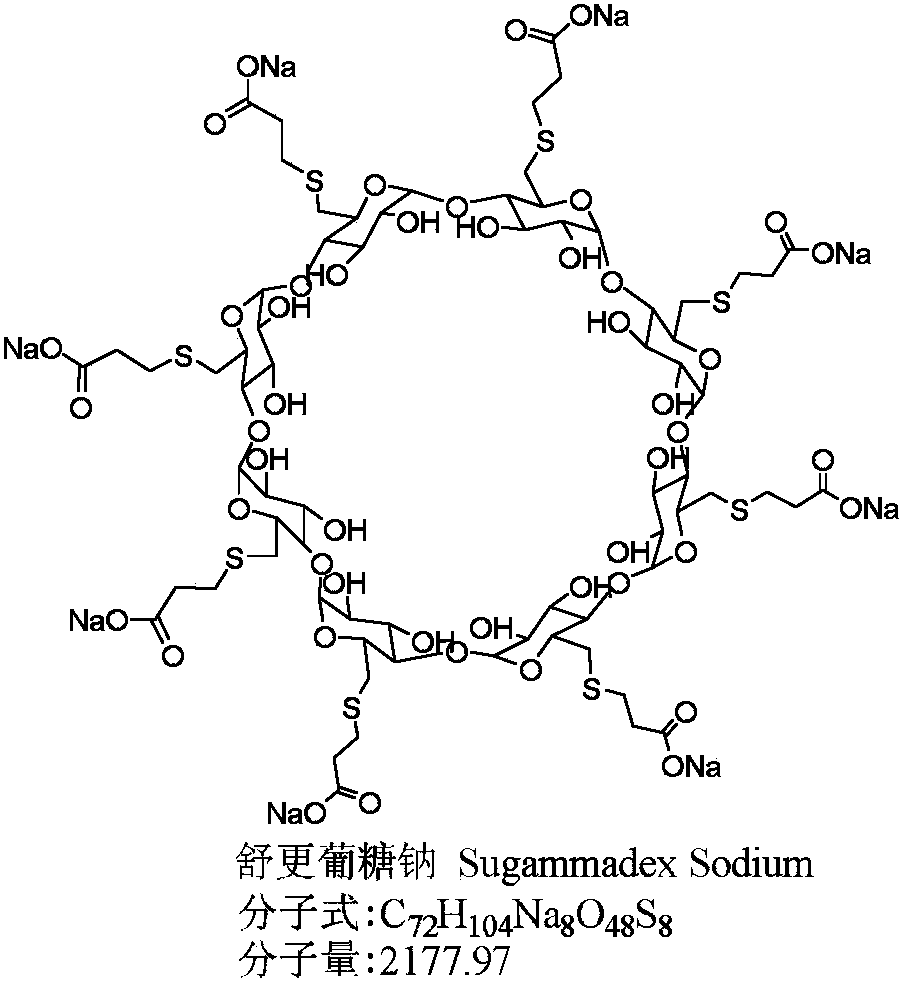
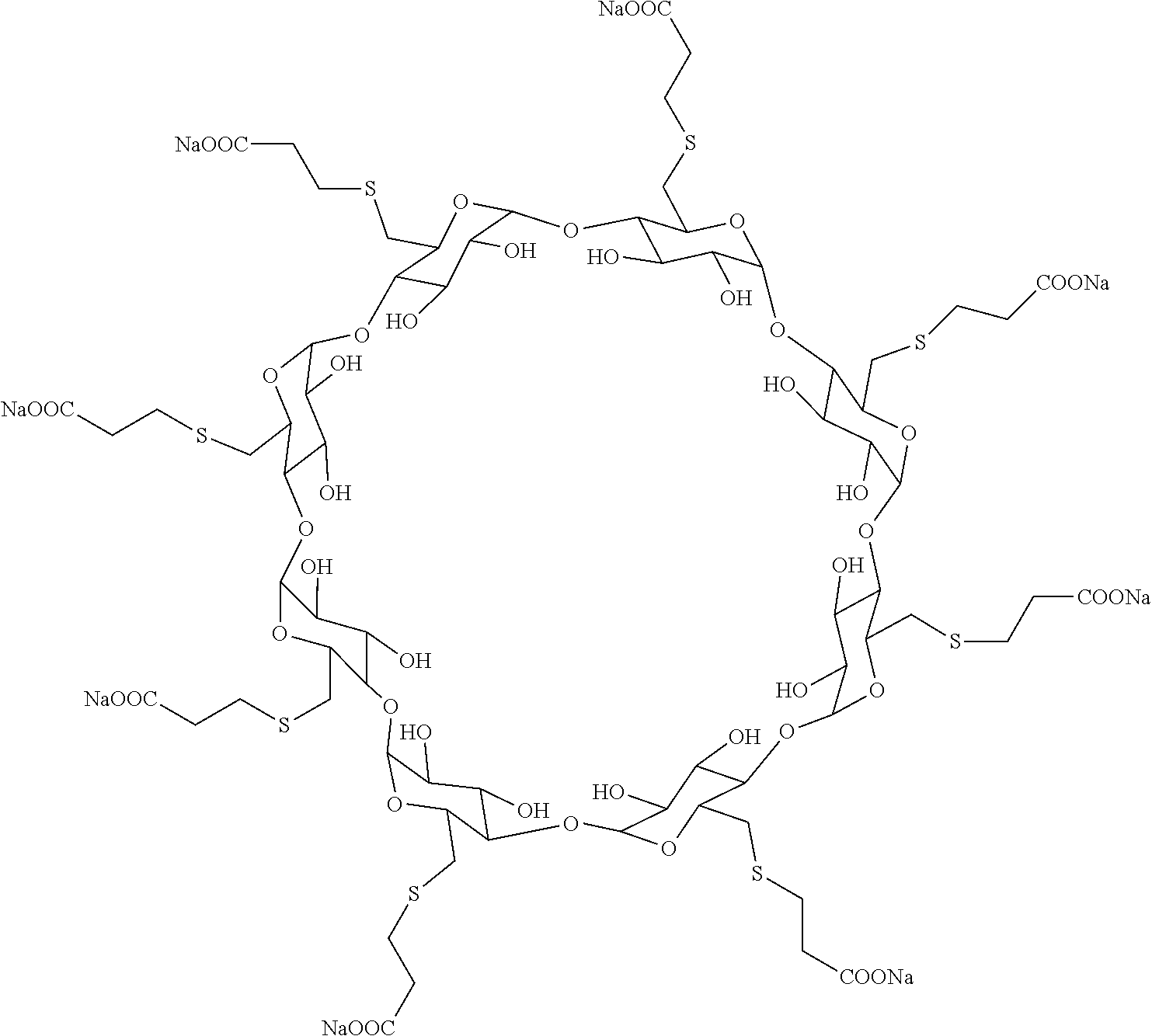
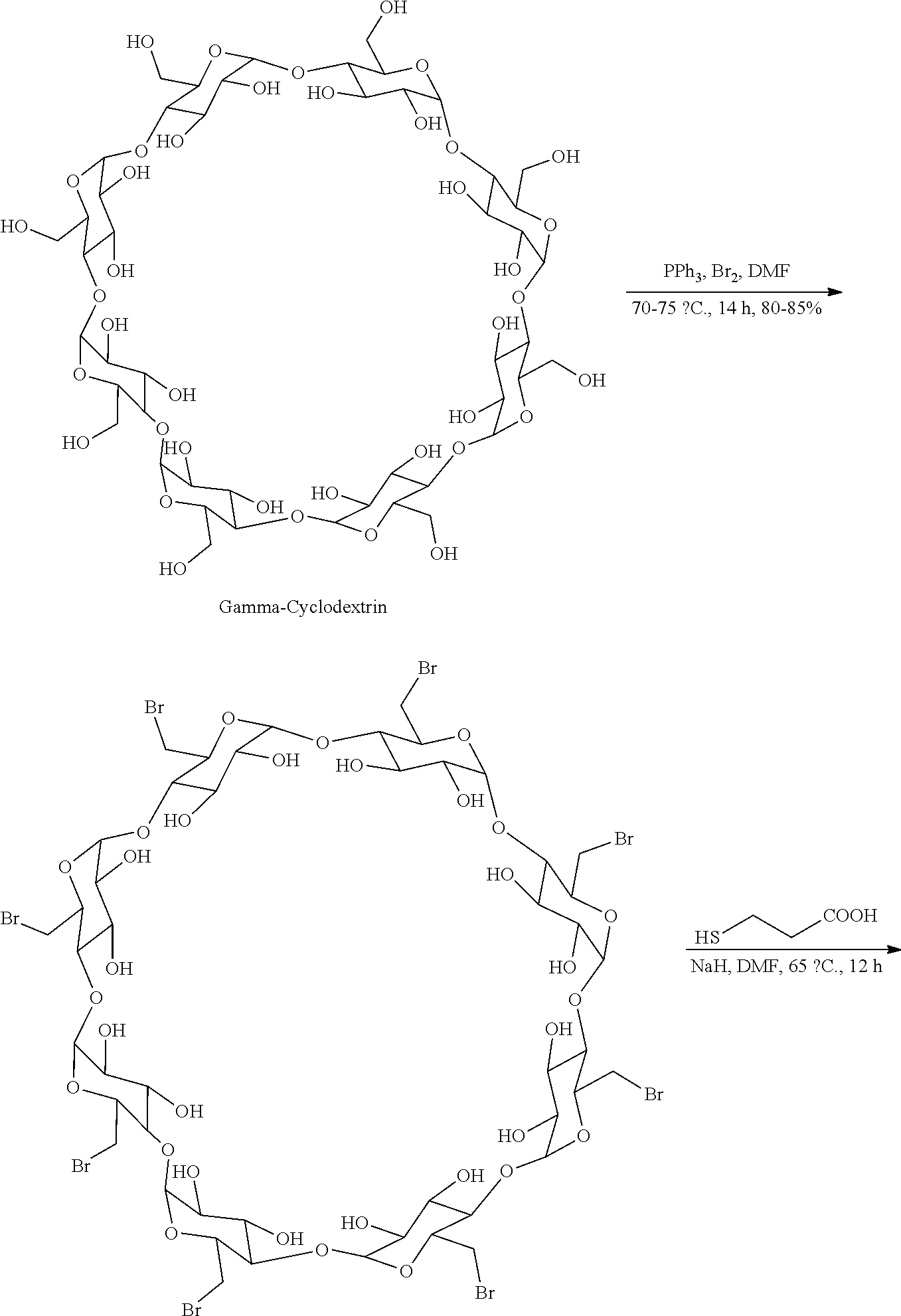
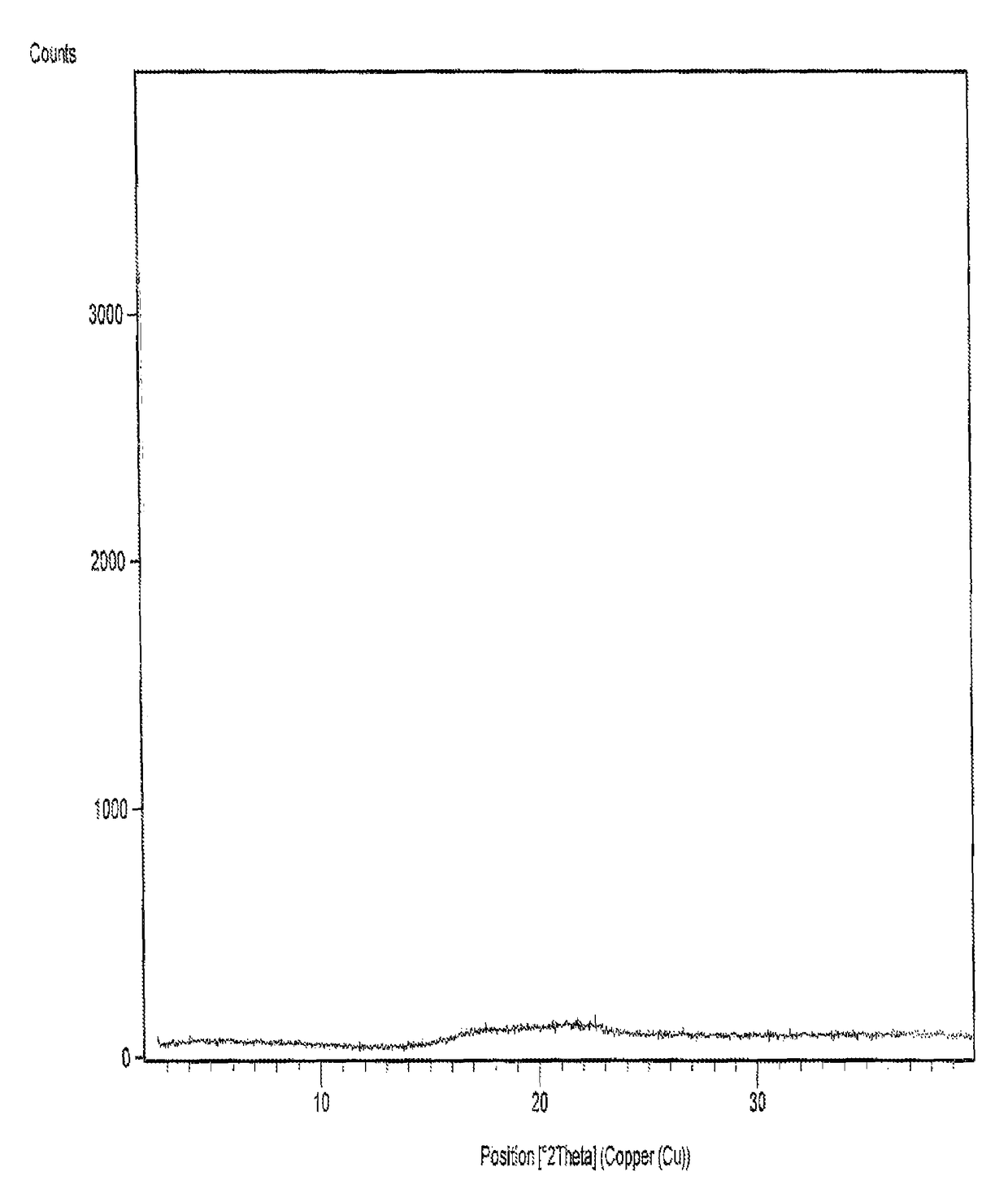
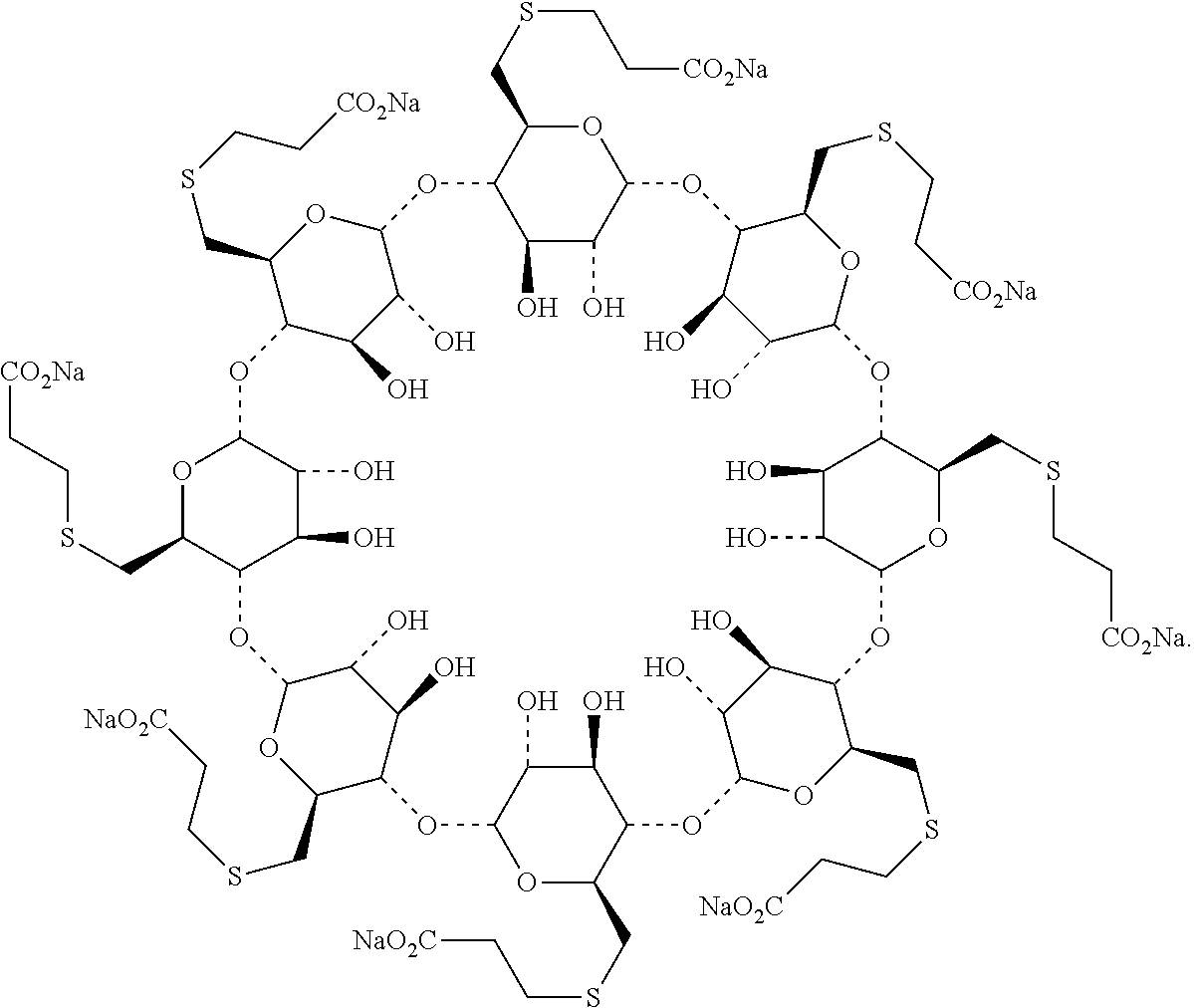
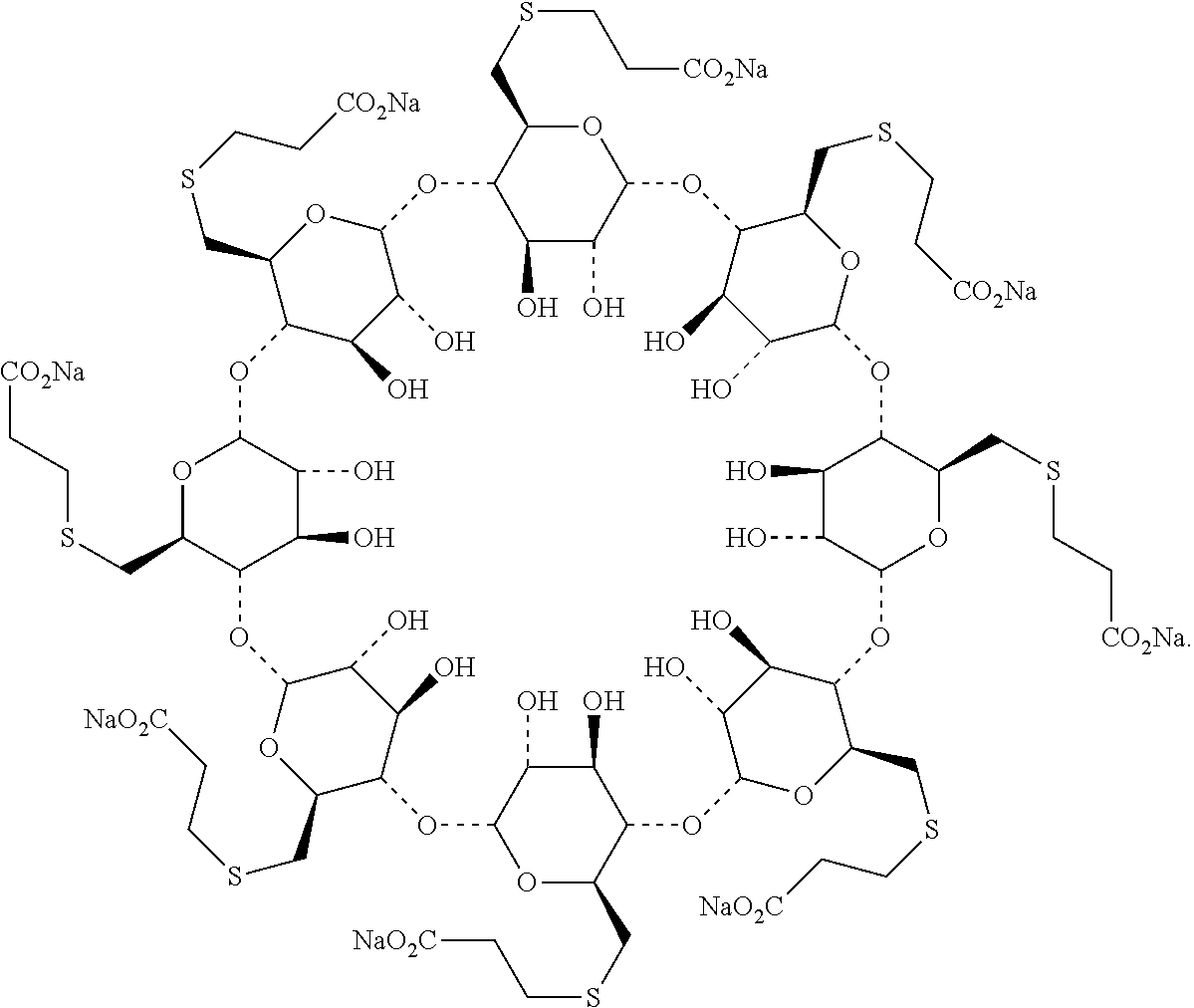
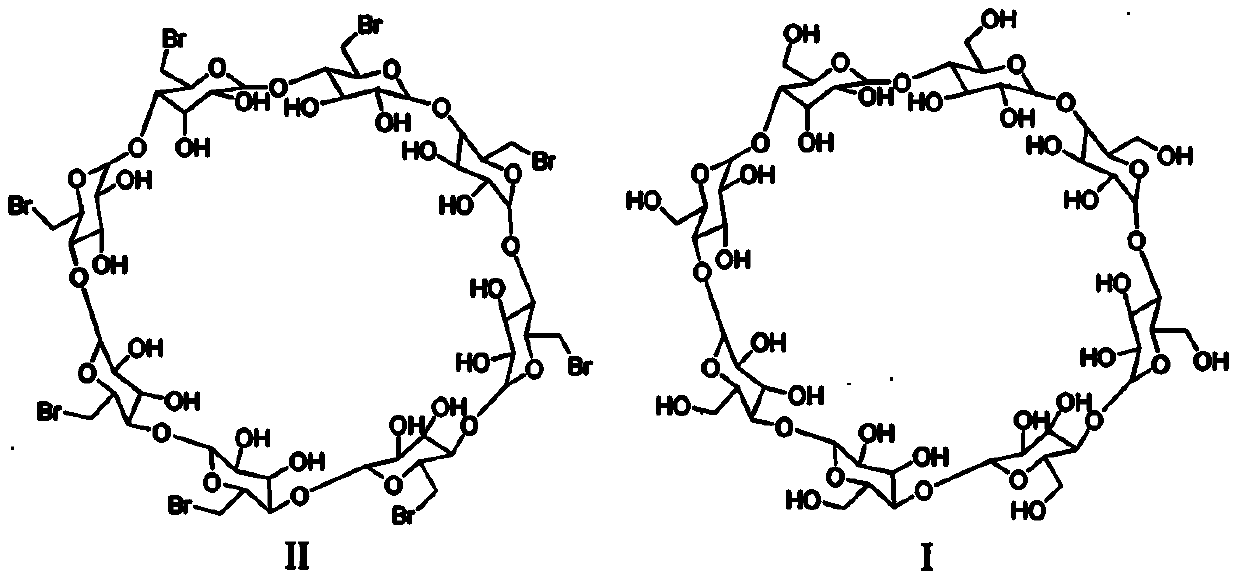
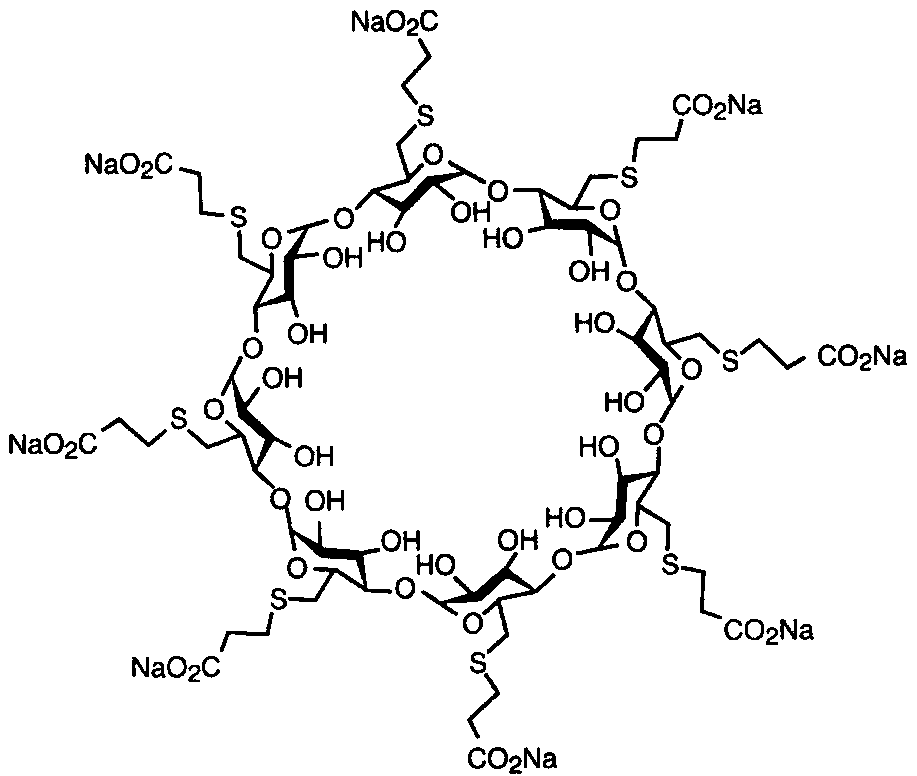
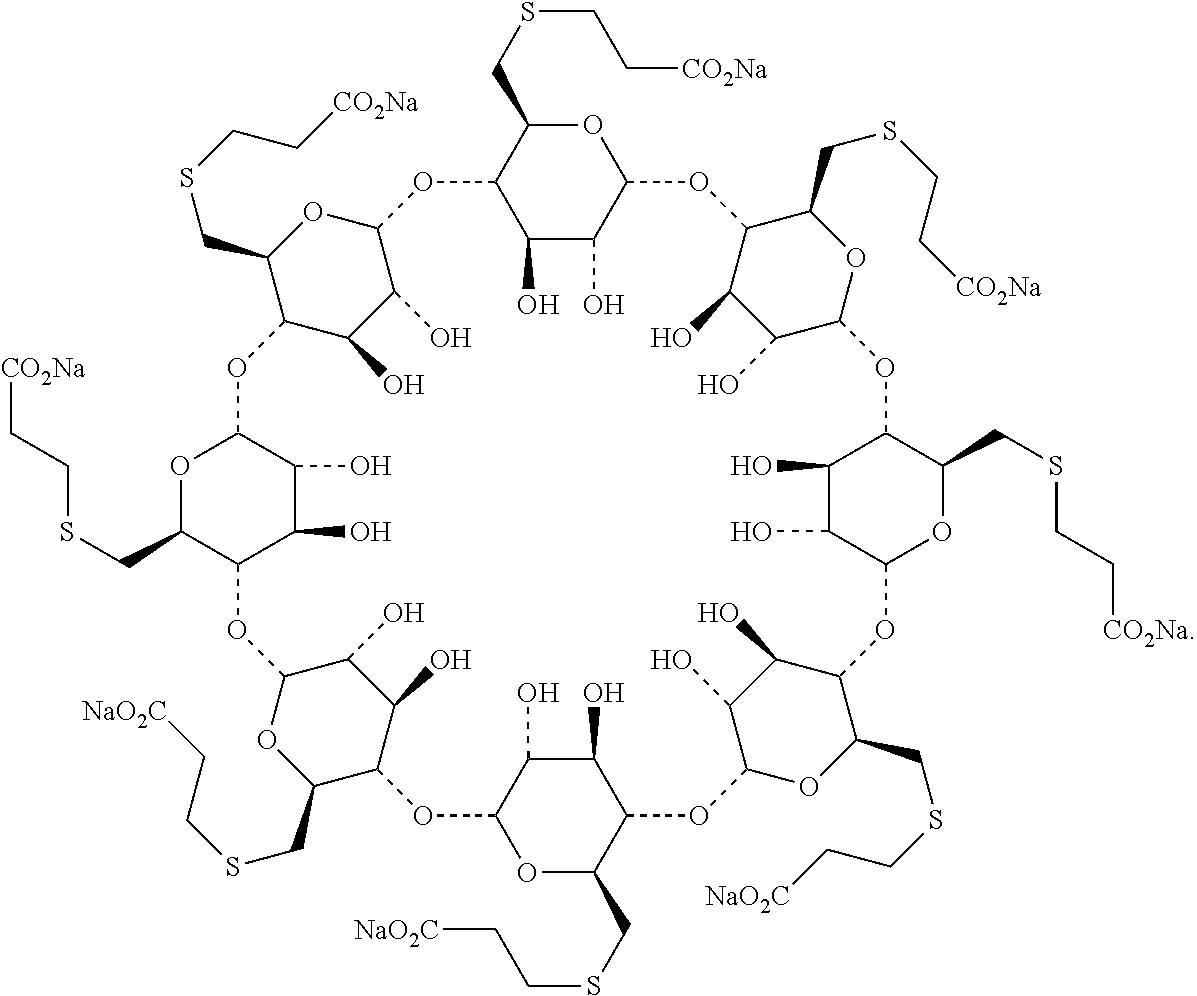
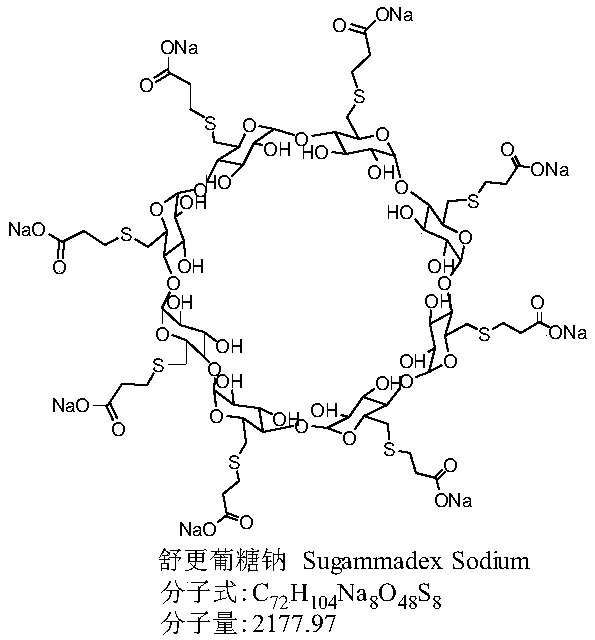
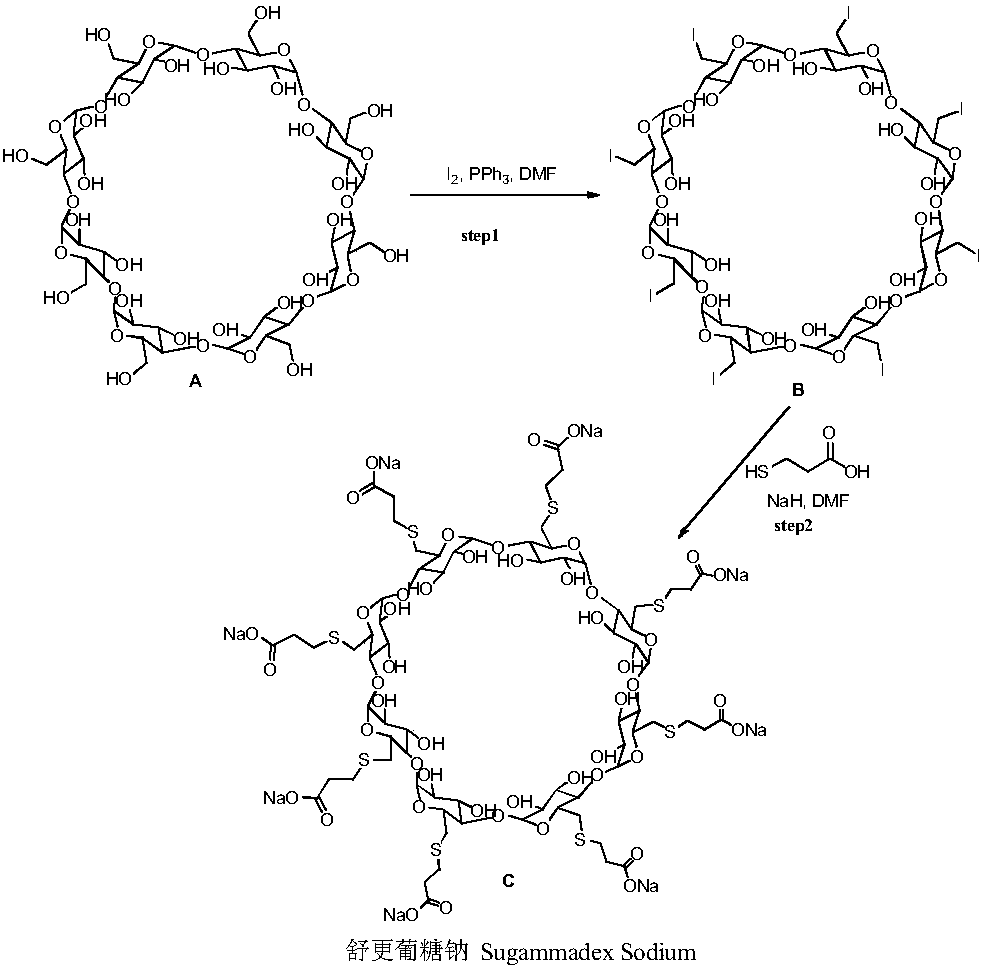
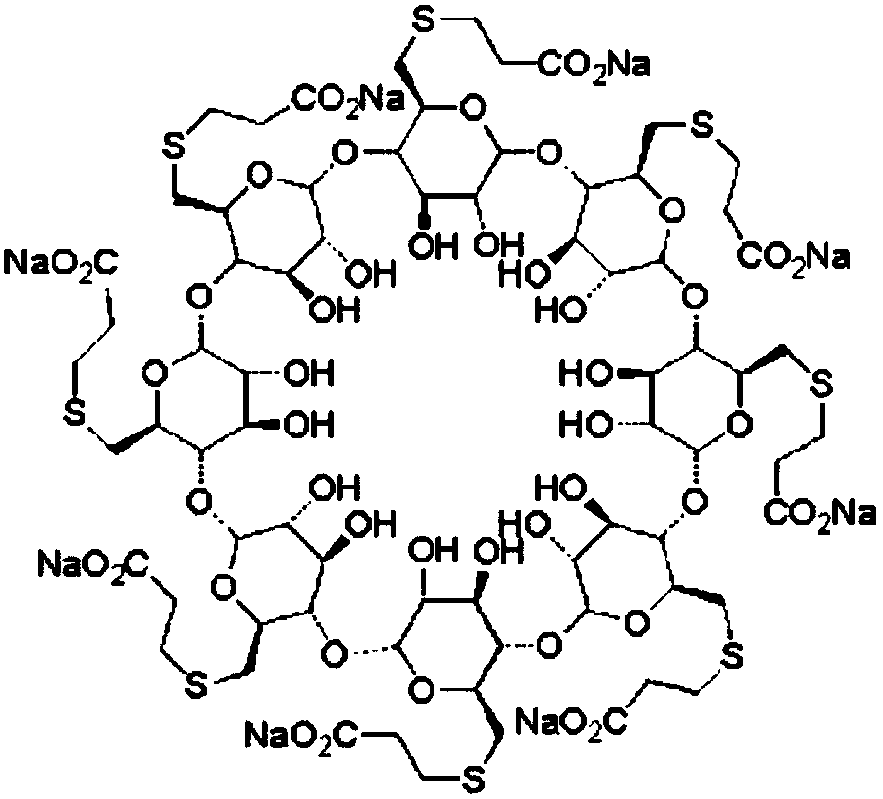
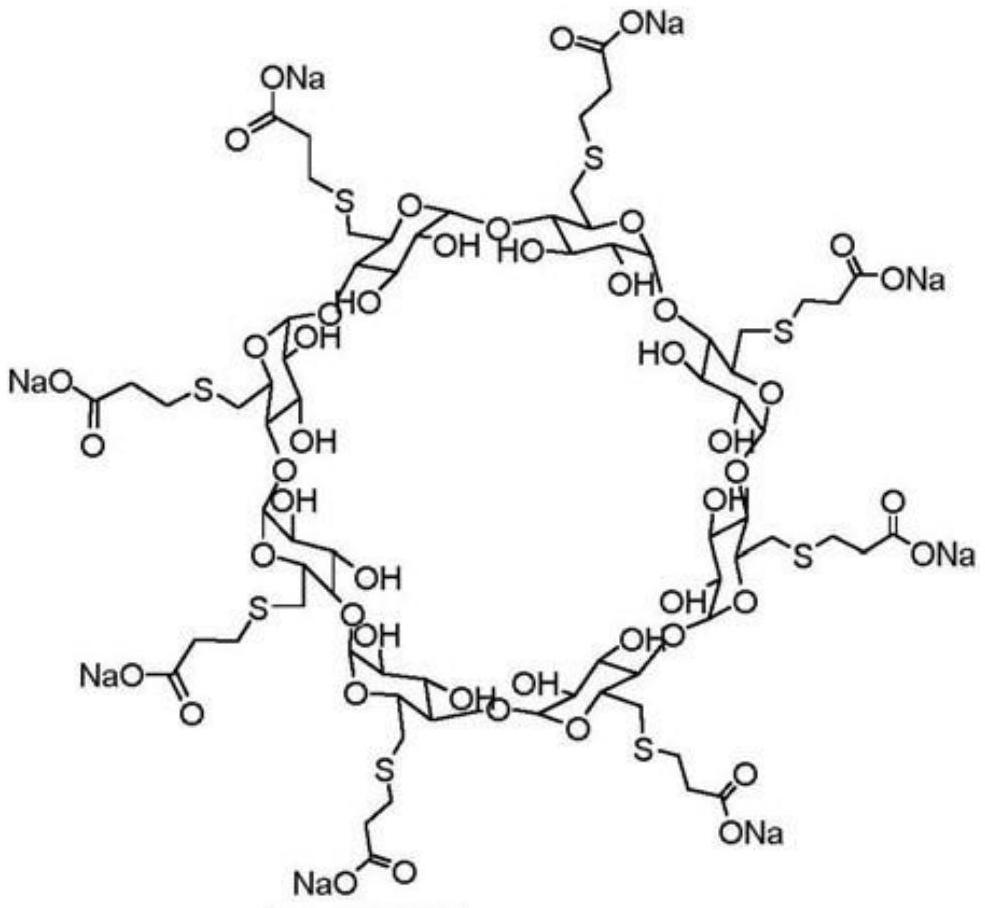
The sodium salt form of the biologically inert, selective relaxant binding agent (SRBA) sugammadex, a modified, anionic gamma cyclodextrin derivative containing a hydrophilic exterior and a hydrophobic core, with neuromuscular blocking drug (NMBD) reversal activity. Upon administration, the negatively charged carboxyl-thio-ether groups of sugammadex selectively and reversibly bind to the positively charged quaternary nitrogen of a steroidal NMBD, which was administered at an earlier time for anesthetic purposes. The encapsulation of the NMBD by sugammadex blocks its ability to bind to nicotinic receptors in the neuromuscular junction and thereby reverses the NMBD-induced neuromuscular blockade. Sugammadex binds rocuronium, vecuronium, and to a lesser extent pancuronium.
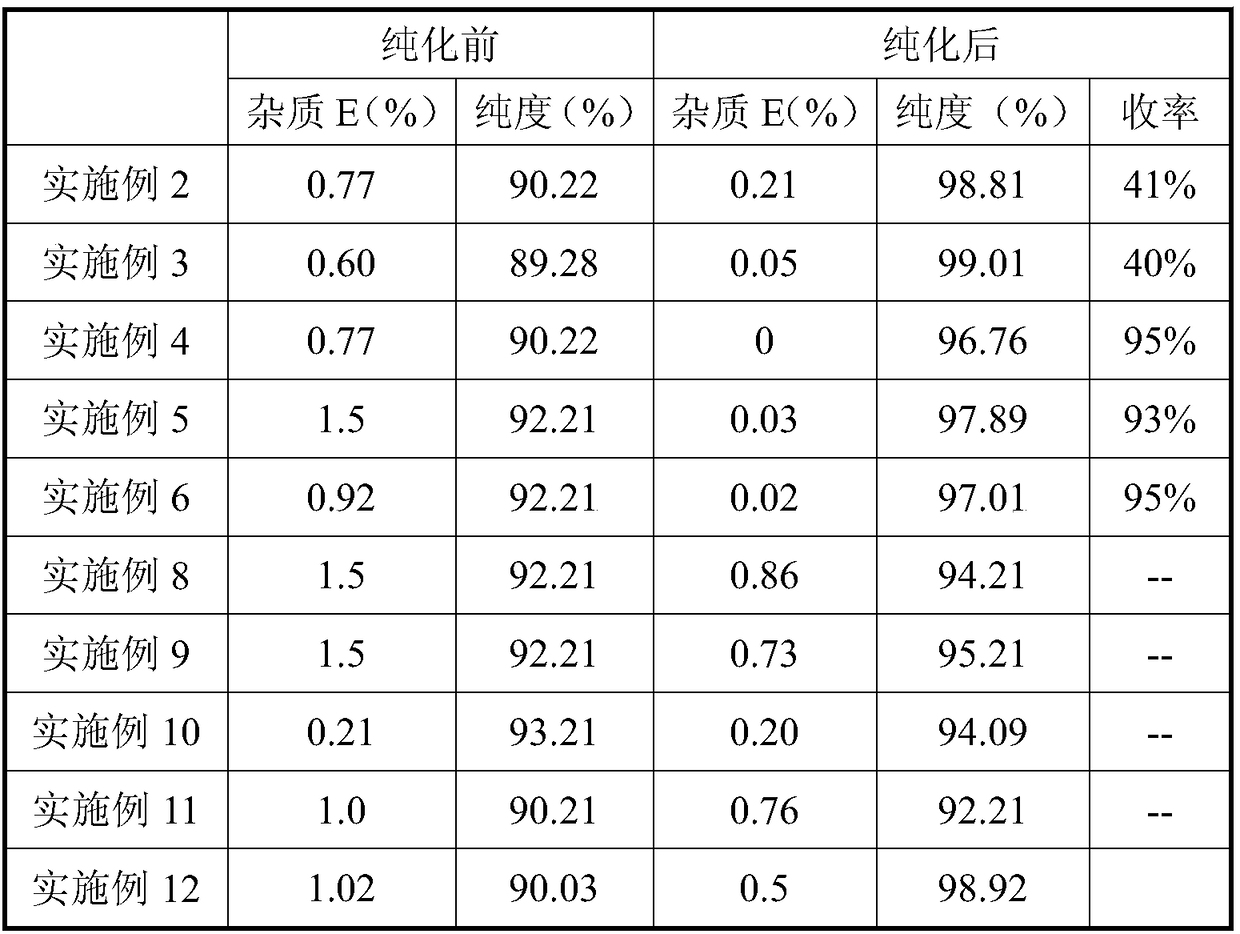
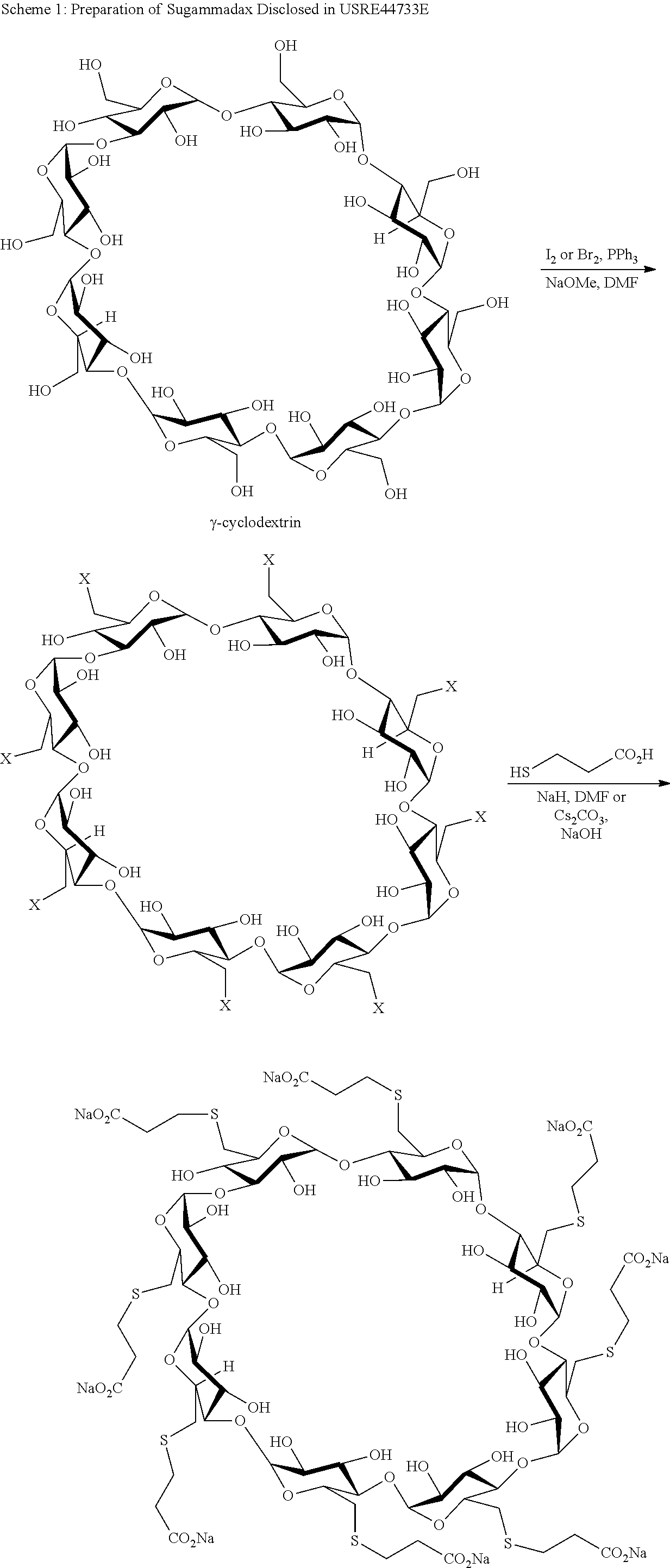
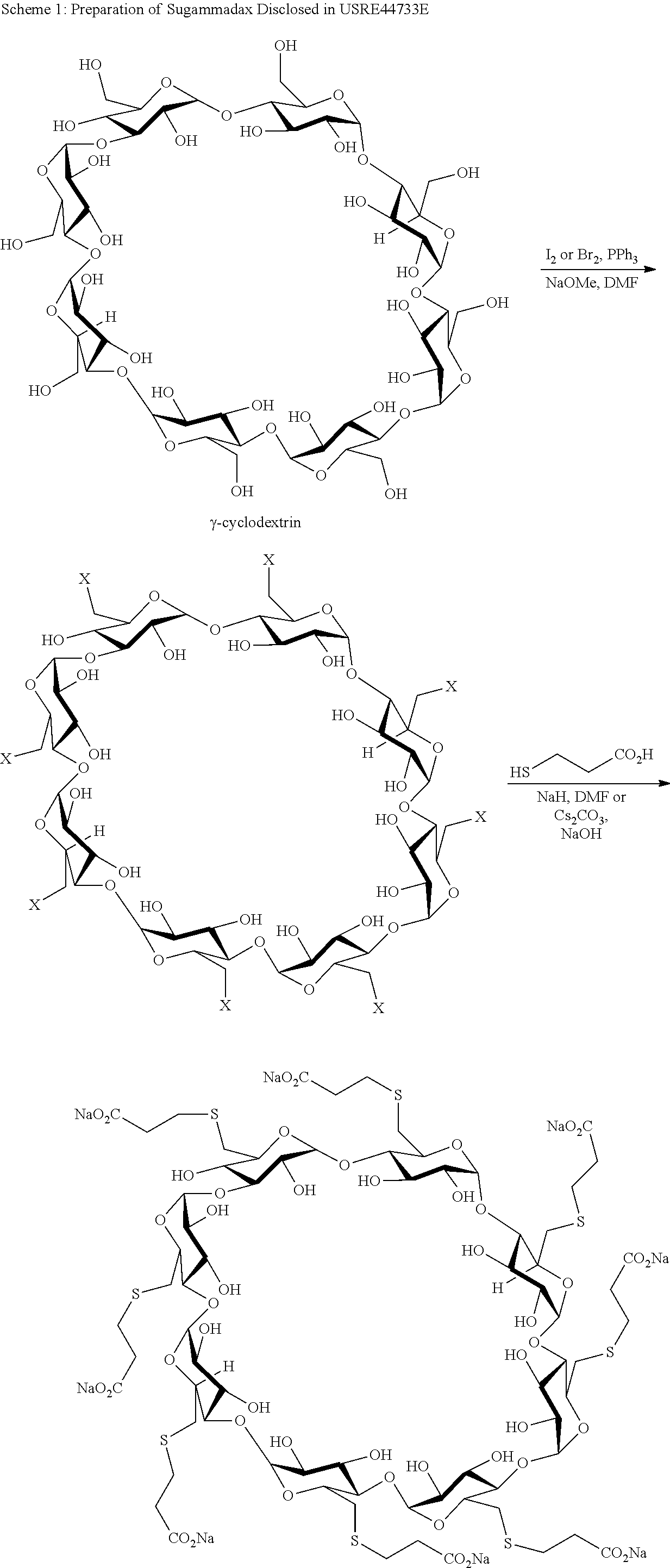
Preparation method for sugammadex sodium
The invention relates to a preparation method for a muscle relaxing antagonistic agent sugammadex sodium. The preparation method comprises the following steps: preparing sulfydryl gamma-cyclodextrin by taking halogenated gamma-cyclodextrin and thiourea as reactants; then, initiating sulfydryl-alkene click reaction by illuminating or using an initiator on sulfydryl gamma-cyclodextrin with acrylic acid or acrylic esters or sodium acrylate so as to prepare high-purity sugammadex sodium in a water phase. According to the preparation method for the sugammadex sodium, the the operation environment is gentle, the yield is increased, and the purifying process for the final product sugammadex sodium is simpler.
Owner:SHANDONG BINZHOU ZHIYUAN BIO TECH CO LTD
Method for purifying sugammadex sodium
The invention discloses a method for purifying sugammadex sodium. The method for purifying sugammadex sodium comprises the steps of hydrolyzing a mmadex ammonium salt under acidic conditions, and carrying out a salt forming reaction with suga sodium hydroxide, thereby obtaining sugammadex sodium. Compared with the prior art, the method provided by the invention has the advantages that the purity of the sugammadex sodium finished product can be remarkably improved; dialysis and column chromatography are not used during purification; and the yield and purity of sugammadex sodium can be remarkably improved by only adopting simple steps such as hydrolysis and recrystallization, so that the method is more applicable to industrial production, and the production efficiency is increased.
Owner:SUZHOU NHWA PHARM RES CO LTD +1
Method for purifying sugammadex sodium
ActiveCN109021147AGood purification effectGood for maintaining stabilityOrganic active ingredientsMuscular disorderPurification methodsImpurity
The invention discloses a method for purifying sugammadex sodium. The method is characterized in that a sugammadex sodium crude product is subjected to dissociation in an organic acid-organic alkali-reducing agent mixture system into sugammadex acid, the free condition is mild, the product stability is high, and the purification effect of the sugammadex acid is better, and after further recrystallization purification and salt formation, the high-purity sugammadex sodium can be obtained. Both the dissociation and recrystallization steps of the purification method introduce a reducing agent so that the reaction system has oxidation resistance, and effectively controls the formation of oxidized impurities, the reagent is easy to obtain, and the operation is simple and safe, compared with theprior art, a large amount of activated carbon, multitime long-time dialysis and preparation of HPLC are employed, the sugammadex sodium is purified, the method is more suitable for industrial amplification, and provides bulk drugs of sugammadex sodium which can meet the purity requirements and impurity limitation for preparation production. The invention further discloses high-purity sugammadex sodium with content being more than 99.5% and single impurity of less than 0.1%, and a preparation and use thereof.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Method for refining sugammadex sodium
The invention discloses a method for re-crystallizing, refining and purifying sugammadex sodium. The method comprises the following steps: adding a protective agent to crude sugammadex sodium, and performing re-crystallizing under the protection of an inert gas to obtain pure sugammadex sodium, wherein the protective agent is selected from one or a mixture of two or more of mercaptoethanol, mercaptoacetate, mercaptoacetate ester, mercaptopropionate, mercaptopropionate ester, glutathione, cysteine, cystamine, dithioerythritol, dithiothreitol, trisubstituted organophosphorus compounds and trisubstituted organophosphorus compound salts. The method has the advantages of simplicity in operation, high product purity, good economy, and suitableness for industrial production.
Owner:王炳永
Method for refining sugammadex sodium
ActiveCN110627925AHigh purityHigh yieldOrganic active ingredientsMuscular disorderSugammadex SodiumSolvent
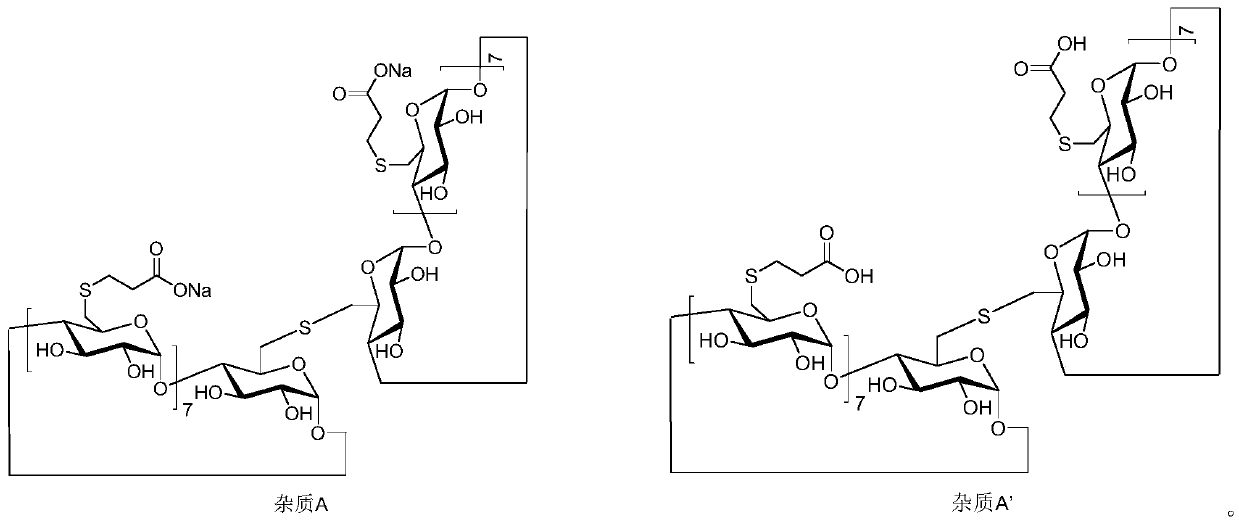
The invention discloses a method for refining sugammadex sodium. The method comprises the following steps: liquid separating and refining a crude sugammadex sodium product in a poor solvent / water system, preferentially separating out and enriching dimer impurities which are difficult to remove into solids to remove, separating (liquid separating and filtering) to obtain a mother liquor, further adding a poor solvent for crystallizing to obtain high-purity sugammadex sodium. According to the refining method provided by the invention, the contents of dimer impurities A and B can be effectively reduced; the total purity of the refined sugammadex sodium is greater than 99.5%, the impurity A is not detected in the finished product, the content of impurity B is less than 0.1%, the content of other single impurity is less than 0.1%, the adopted reagents are all conventional and easy to obtain, the operation is simple and safe, and the method is very suitable for industrial amplification underconventional production conditions.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
An Improved Process for Preparation of Sugammadex Sodium
The present invention provides a process for the preparation of sugammadex sodium involving the step of: reacting 6-perdeoxy-6-per-halo-gamma-cyclodextrin with 3-mercapto propionic acid in the presence of alkali metal alkoxide in an organic solvents. The invention also provides a process for purifying the sugammadex or its pharmaceutically acceptable salts using water and organic solvents.
Owner:NEULAND LABORATORIES LTD
Preparation method for green and environmentally friendly sugammadex sodium
The invention belongs to the field of pharmacy, and especially relates to a preparation method for green and environmentally friendly sugammadex sodium. The preparation method obtains a crude productof sugammadex sodium through preparation for the first time by taking 3-mercaptopropionic acid, alkali and 6-deoxy-6-perhalogeno-gamma-cyclodextrin as raw materials; and high purity sugammadex sodiumcan be obtained after desolvation through recrystallization and column chromatography purification. Compared with the prior art, a reaction solvent of the preparation method can replace organic solvents like N,N-dimethylformamide used by traditional methods by only using water, so that disadvantages of hard recovery and large pollution caused by large amount of high boiling organic solvents can beimproved; and the sugammadex sodium is clean and environmentally friendly, green and efficient, has advantages of being mild in reaction condition, high in product purity, simple in post-treatment step, high in product yield, low in cost and convenient for industrialization, and has good application prospects.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Preparation method of amorphous sugammadex sodium
The invention discloses a preparation method of amorphous sugammadex sodium. The preparation method comprises the following step of selecting a special solvent, or directly concentrating an aqueous solution of sugammadex sodium to the dry state, so as to obtain the amorphous sugammadex sodium product. Compared with the existing preparation method of the special-crystal form type sugammadex sodium,the preparation method has the advantages that the problem of clarification degree due to crystallizing of alcohol solvents is solved, and the quality safety of the finished product of the sugammadexsodium is improved.
Owner:郭辉
Method for preparation of sugammadex sodium
InactiveUS20190062459A1Mercapto/sulfide group formation/introductionOrganic active ingredientsSugammadex SodiumCombinatorial chemistry
Owner:FORMOSA LAB
Purifying method of sugammadex sodium
The invention provides a purifying method of sugammadex sodium. The purifying method specially comprises the following steps: putting a sugammadex sodium crude product to a chromatography column usingsilica gel C18 as a filling, and performing purifying, wherein the purity of the obtained sugammadex sodium is greater than 99%. Through adoption of the purifying method disclosed by the invention, the quality of the products can be guaranteed, and the purifying method is suitable for process scaled production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Processes for preparation of sugammadex and intermediates thereof
ActiveUS20180171033A1High yieldHigh purityOrganic active ingredientsAntinoxious agentsSugammadex SodiumMedicinal chemistry
The present invention relates to a process for preparation of 6-perdeoxy-6-per-chloro gamma-cyclodextrin which is a key intermediate useful in the synthesis of Sugammadex sodium. The present invention further relates to a process for preparation and purification of Sugammadex sodium.
Owner:ALAPARTHI LAKSHMI PRASAD
Process for preparation of sugammadex sodium
Owner:NEULAND LABORATORIES LTD
Method for preparing sugammadex sodium
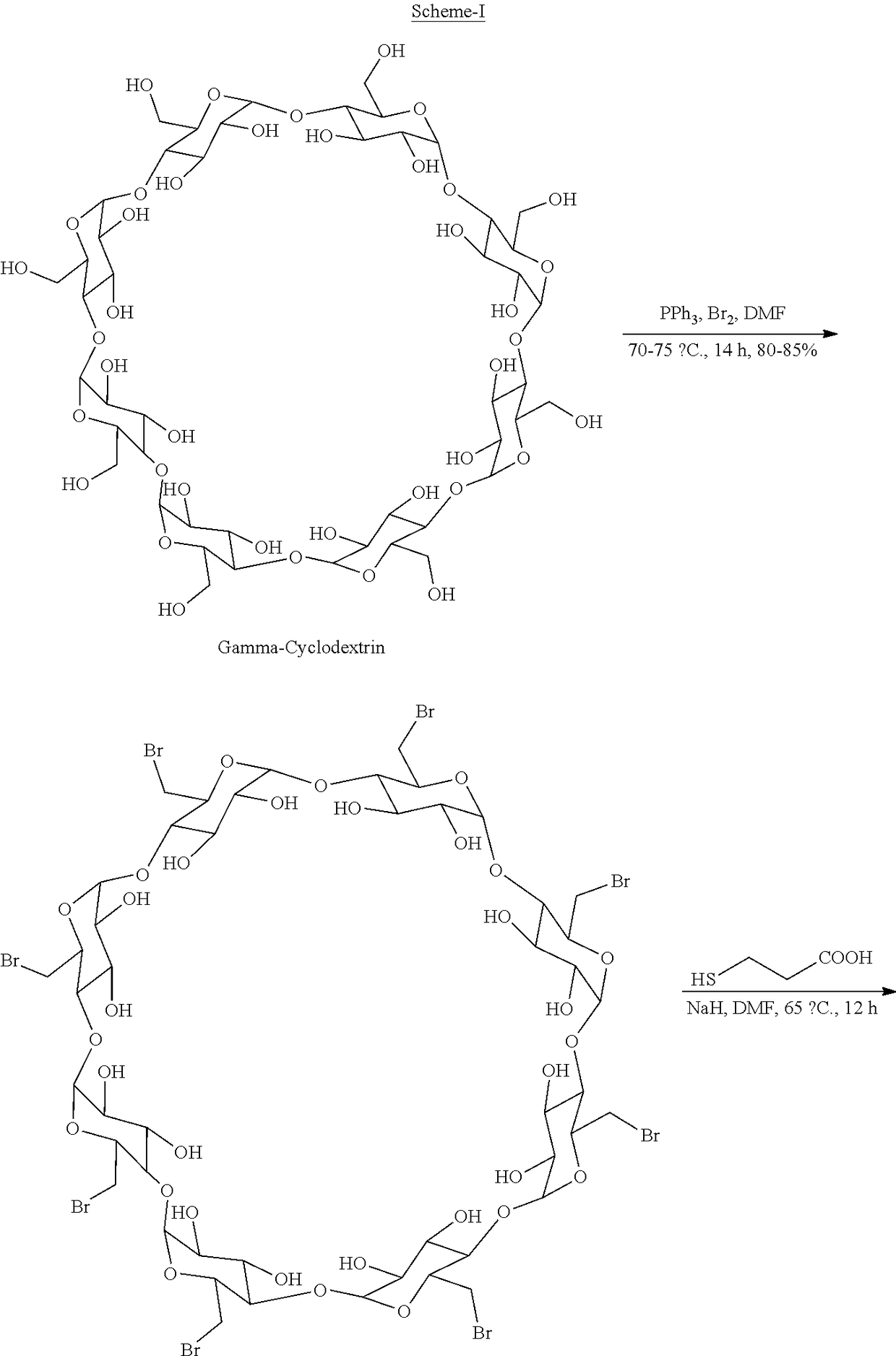
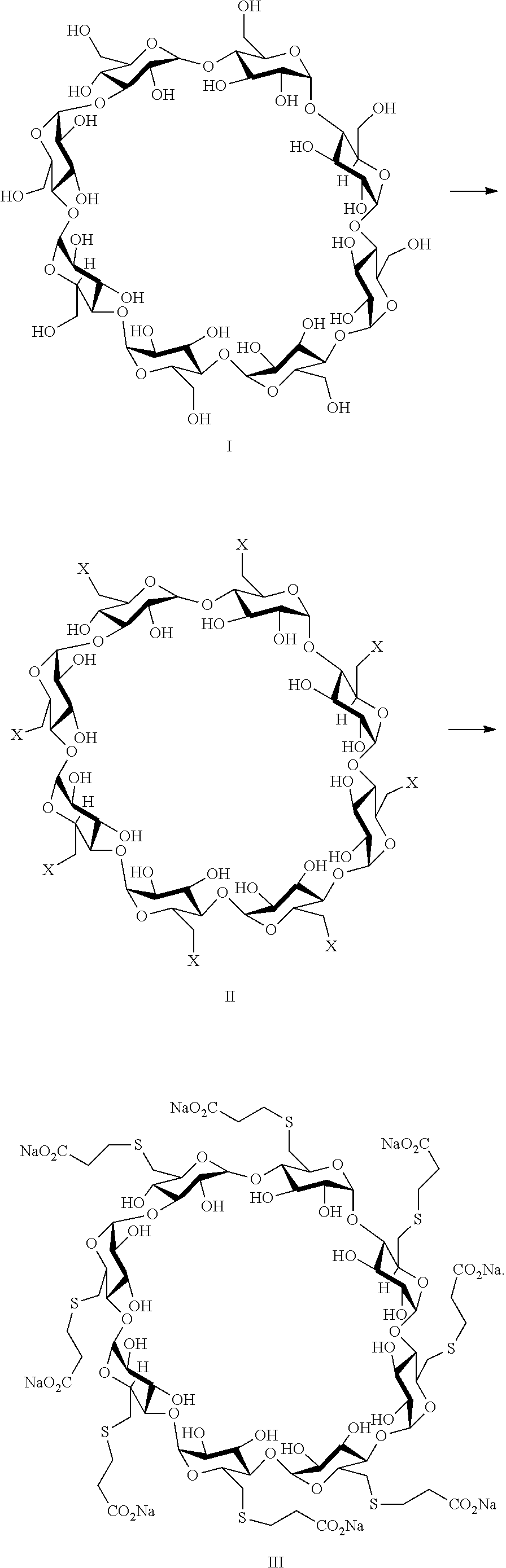
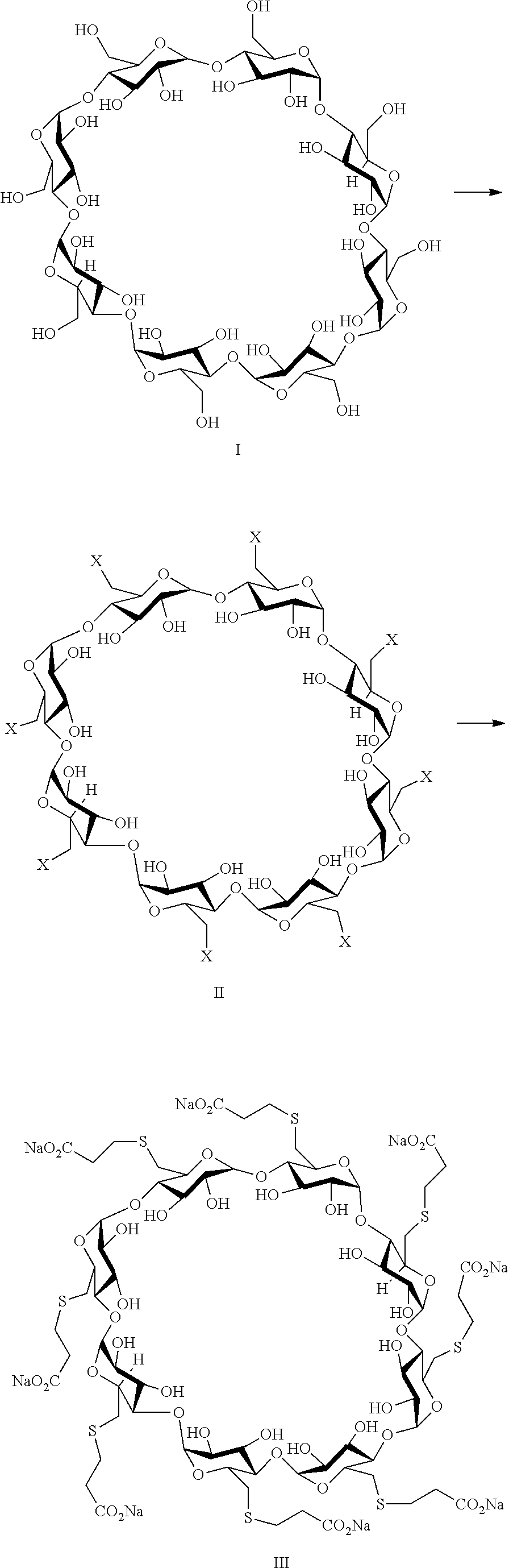
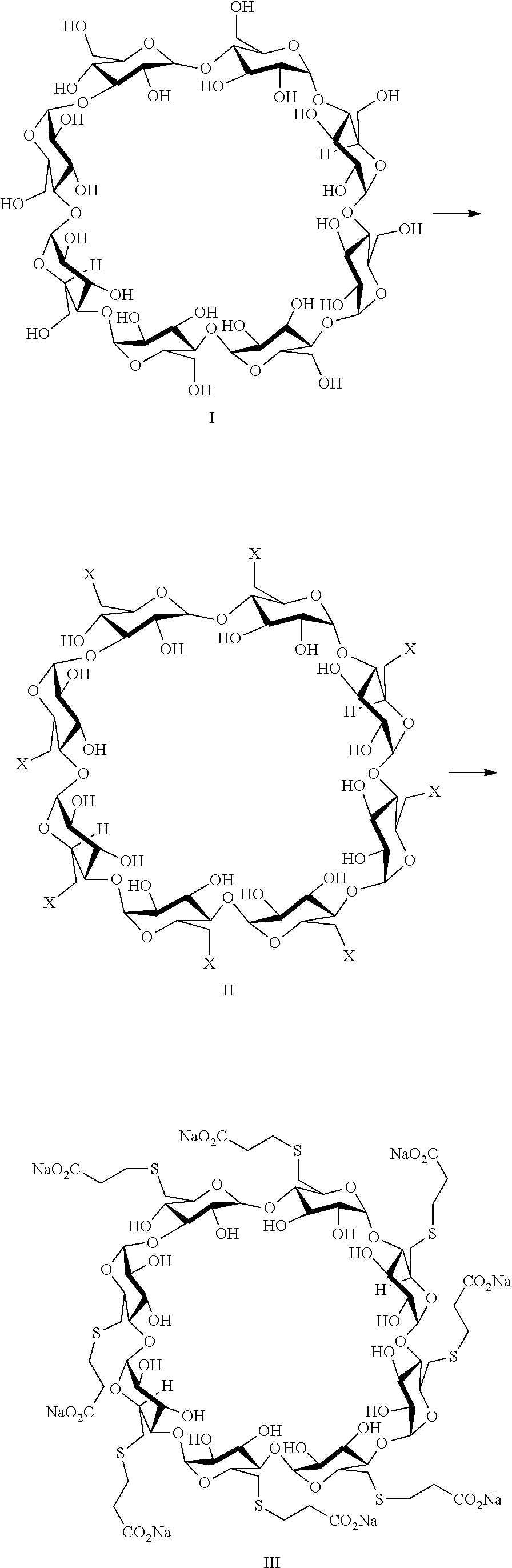
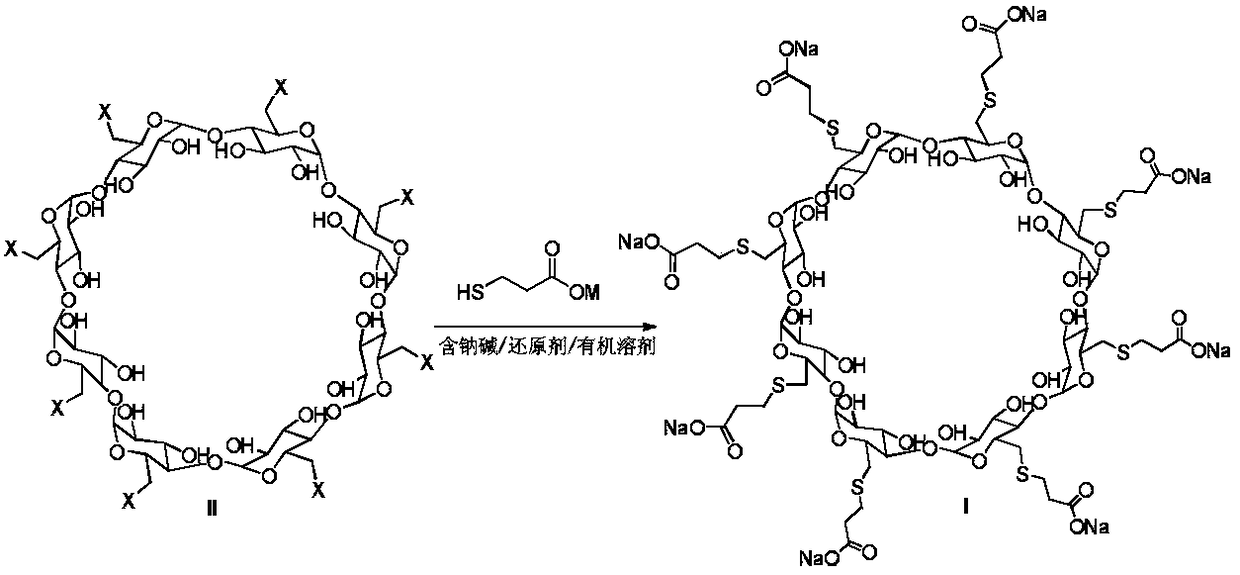
A process for preparing sugammadex sodium comprising:reacting a γ-cyclodextrin of formula I with a halogenating agent in the presence of N-methyl-2-pyrrolidone to provide a compound of formula II; andreacting the compound of formula II with 3-mercapto propionic acid in the presence of a sodium base and an organic solvent to provide sugammadex sodium formula III:
Owner:SCINOPHARM TAIWAN LTD
Sugammadex sodium impurity and preparing method thereof
The invention relates to the technical field of drug synthesis, in particular to a sugammadex sodium impurity and a preparing method thereof. A chemical structural formula of the impurity is shown ina formula I. The preparing method comprises the following steps that 1, sugammadex sodium is put in an aqueous solution, and after a reaction is carried out for a period of time at a certain temperature in the presence of an oxidizing agent, oxidation is conducted to generate a compound with the formula I, wherein the compound is a mixture of a compound II and a compound III; 2, the obtained mixture of the compound II and the compound III is subjected to preparative separation to obtain the single compound II and the single compound III. The preparing method has the advantages that operation is convenient, the reaction condition is mild and controllable, the stability of a reaction is high, and a reaction product is high in yield and purity; moreover, the compound with the formula I can provide an impurity reference substance which meets the requirement for quality control over the sugammadex sodium.
Owner:CHANGZHOU YABANG PHARMA
Preparation technology of sugammadex sodium
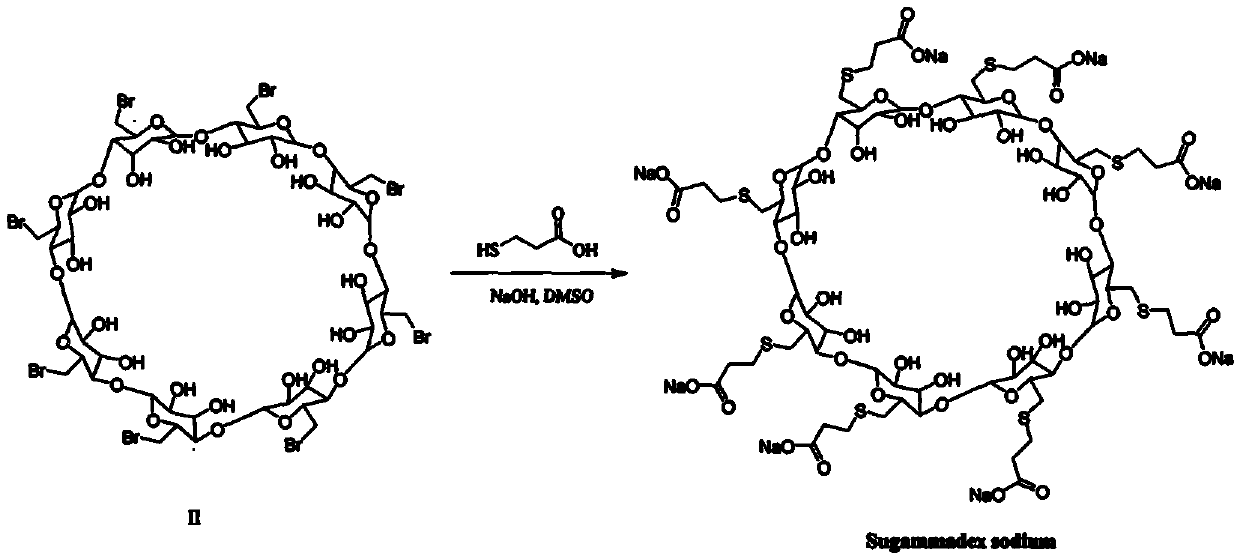
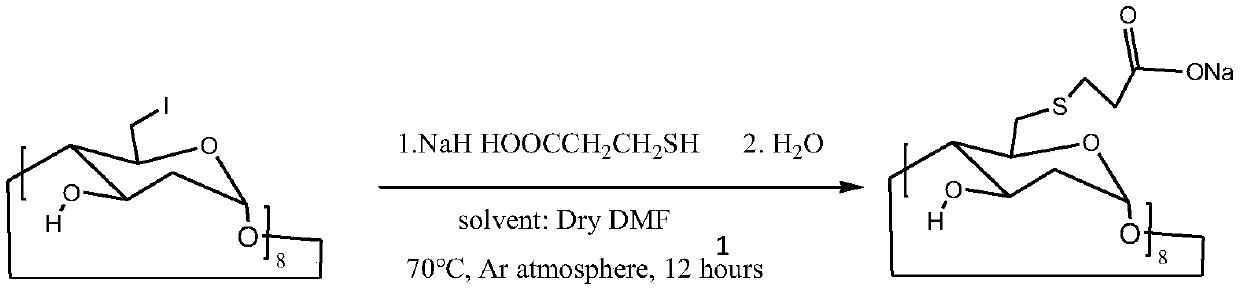
The invention provides a method for preparing sugammadex sodium, which is simple in technology, high in purity and high in yield. The method comprises the following step: enabling per(6-deoxy-6-iodo)gamma-cyclodextrin to react with 3-thiohydracrylic acid under the existence of sodium hydride and BHT (2,6-ditert butyl-4-methylphenol), thus obtaining per[6-deoxy-6-(2-carboxyl)sulfo]gamma-cyclodextrin sodium salt, thus the sugammadex sodium.
Owner:BRIGHTGENE PHARMA
Method for preparing sugammadex sodium
A process for preparing sugammadex sodium comprising:reacting a γ-cyclodextrin of formula I with a halogenating agent in the presence of N-methyl-2-pyrrolidone to provide a compound of formula II; andreacting the compound of formula II with 3-mercapto propionic acid in the presence of a sodium base and an organic solvent to provide sugammadex sodium formula III:wherein X in formula II is halo.
Owner:SCINOPHARM TAIWAN LTD
Processes for preparation of Sugammadex and intermediates thereof
ActiveUS10494450B2High yieldHigh purityOrganic active ingredientsAntinoxious agentsSugammadex SodiumMedicinal chemistry
The present invention relates to a process for preparation of 6-perdeoxy-6-per-chloro gamma-cyclodextrin which is a key intermediate useful in the synthesis of Sugammadex sodium. The present invention further relates to a process for preparation and purification of Sugammadex sodium.
Owner:ALAPARTHI LAKSHMI PRASAD
Preparation method of Sugammadex sodium and intermediate thereof
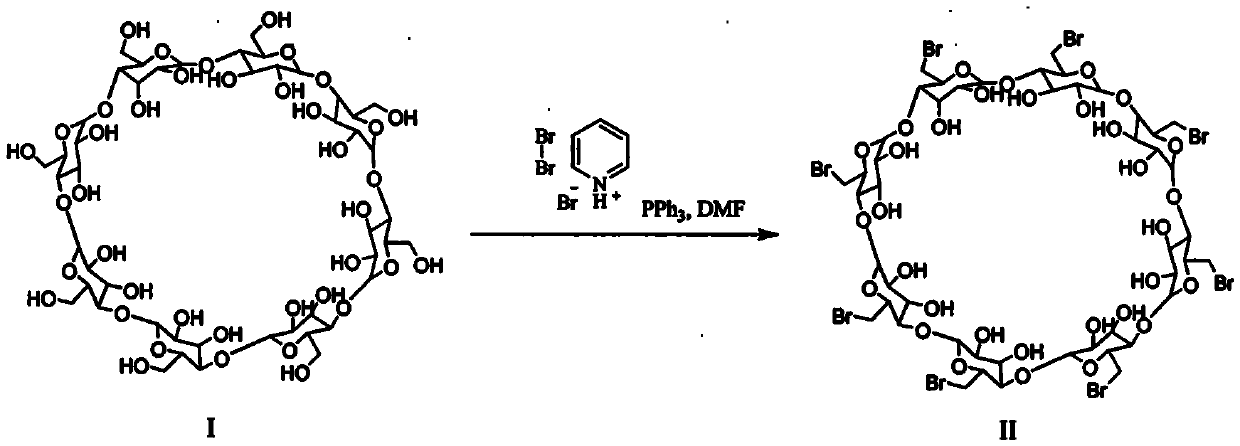
The present invention provides a preparation method of Sugammadex sodium and an intermediate thereof. The method comprises a bromination reaction of a compound of a formula (I) with a brominating reagent, wherein the brominating agent is selected from pyridinium tribromide. The method has the advantages of simple process, high yield, high purity and low pollution, and is suitable for industrial production.
Owner:北京晨光同创医药研究院有限公司
Method for purifying sugammadex sodium
The invention belongs to the technical field of medicine synthesis, and particularly relates to a method for purifying sugammadex sodium. The method comprises the following steps: a sugammadex sodiumcrude product is subjected to acetone pulping under an acidic condition and hydrolysis to obtain sugammadex acid, and finally the sugammadex acid and sodium hydroxide are salified to obtain the sugammadex sodium. Compared with the prior art, the method has the advantages as follows: the purity of the sugammadex sodium finished product can be remarkably improved, dialysis and column chromatographyare not needed in the purification process, yield and purity of sugammadex sodium can be improved only through simple hydrolysis, the method is more suitable for industrial production, and the production efficiency is improved.
Owner:XUZHOU COLLEGE OF INDAL TECH
Sugammadex sodium refining method capable of reducing purification loss rate
ActiveCN110627927AHigh yieldHigh purityOther chemical processesAlkali metal oxides/hydroxidesLoss rateOrganic solvent
The invention discloses a sugammadex sodium refining method capable of reducing the purification loss rate. The method utilizes magnetic polymer microspheres with PVA / sulfydryl group co-modified surfaces to purify acidified sugammadex sodium. The method provided by the invention achieves very high purification yield and purity, has mild purification conditions, avoids side reactions, reduces of the dosage of organic solvent, has environment-friendly process, is low in purification cost, and has substantially reduced purification loss rate.
Owner:深圳市祥根生物有限公司
Method for preparing sugammadex sodium
A process for preparing sugammadex sodium comprising:reacting a γ-cyclodextrin of formula I with triphosgene in the presence of N-methyl-2-pyrrolidone to provide a compound of formula II; andreacting the compound of formula II with 3-mercapto propionic acid in the presence of a sodium base and an organic solvent to provide sugammadex sodium formula III:wherein X in formula II is chloro.
Owner:SCINOPHARM TAIWAN LTD
Method for preparation of sugammadex sodium
InactiveUS10233263B1Mercapto/sulfide group formation/introductionOrganic active ingredientsSugammadex SodiumEngineering
Owner:FORMOSA LAB
Method for removing element impurities and pigments in sugammadex sodium refined product
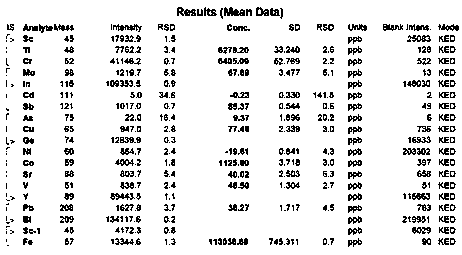
The invention discloses a method for removing element impurities and pigments in a sugammadex sodium refined product. The method comprises steps: dissolving the refined sugammadex sodium product intoan aqueous solution; dropwise adding a certain amount of a poor solvent to separate out a small part of sugammadex sodium and a large part of element impurities and pigments in the solution, separating the precipitate, collecting sugammadex sodium contained in an upper mother liquor, and obtaining a product with the large part of element impurities removed through a direct concentration or solventcrystallization method. The obtained product is light in color, the content of element impurities is remarkably reduced, the requirement of ICH for the limit of the element impurities is met, and themethod is easy, convenient and safe to operate, good in economical efficiency and suitable for industrial production.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Method for preparing sugammadex sodium
The invention discloses a method for preparing sugammadex sodium. The method is characterized in that perhalogeno gamma-cyclodextrin and 3-mercaptopropionic acid or / and a sodium salt thereof are subjected to a replacement reaction in a sodium base-reducing agent-organic solvent system for preparing sugammadex sodium, the method effectively controls the formation of process impurities with similarstructure of sugammadex sodium from a reaction process, and obtains high-yield and high-purity product after simple post-treatment. The invention further provides a method for purifying of a crude product of the sugammadex sodium and conversion of impurities to the target product in a one-step reaction, which is simple, efficient and wasteful, the method improves the yield and purity from variousaspects, and solves the problems of complex operation, high requirements on instruments and low yield in the prior art method, and is suitable for industrial mass production of the sugammadex sodium.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
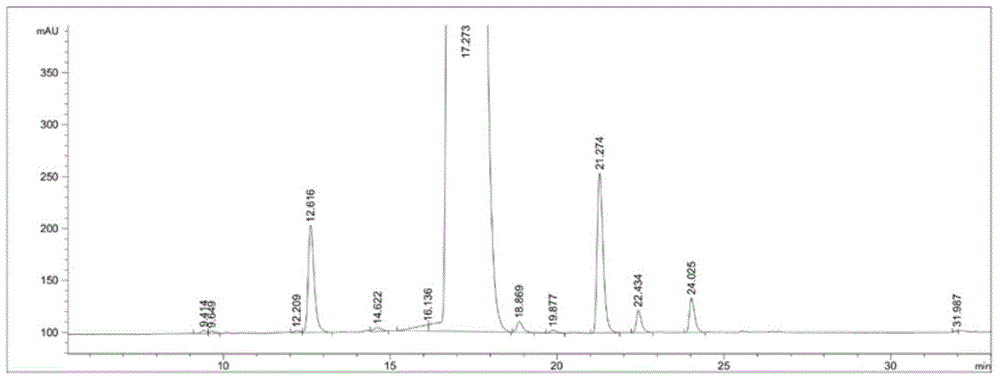
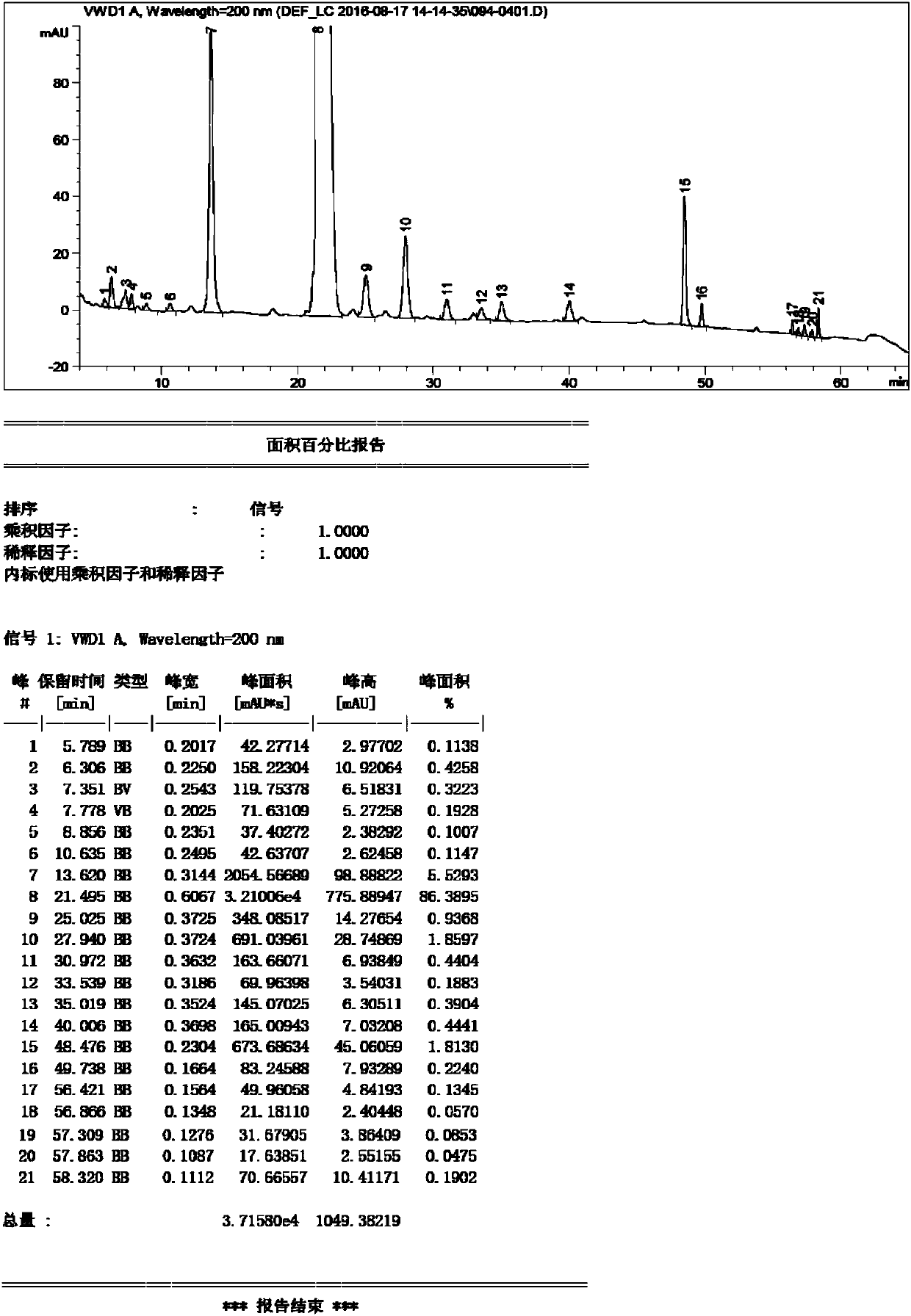
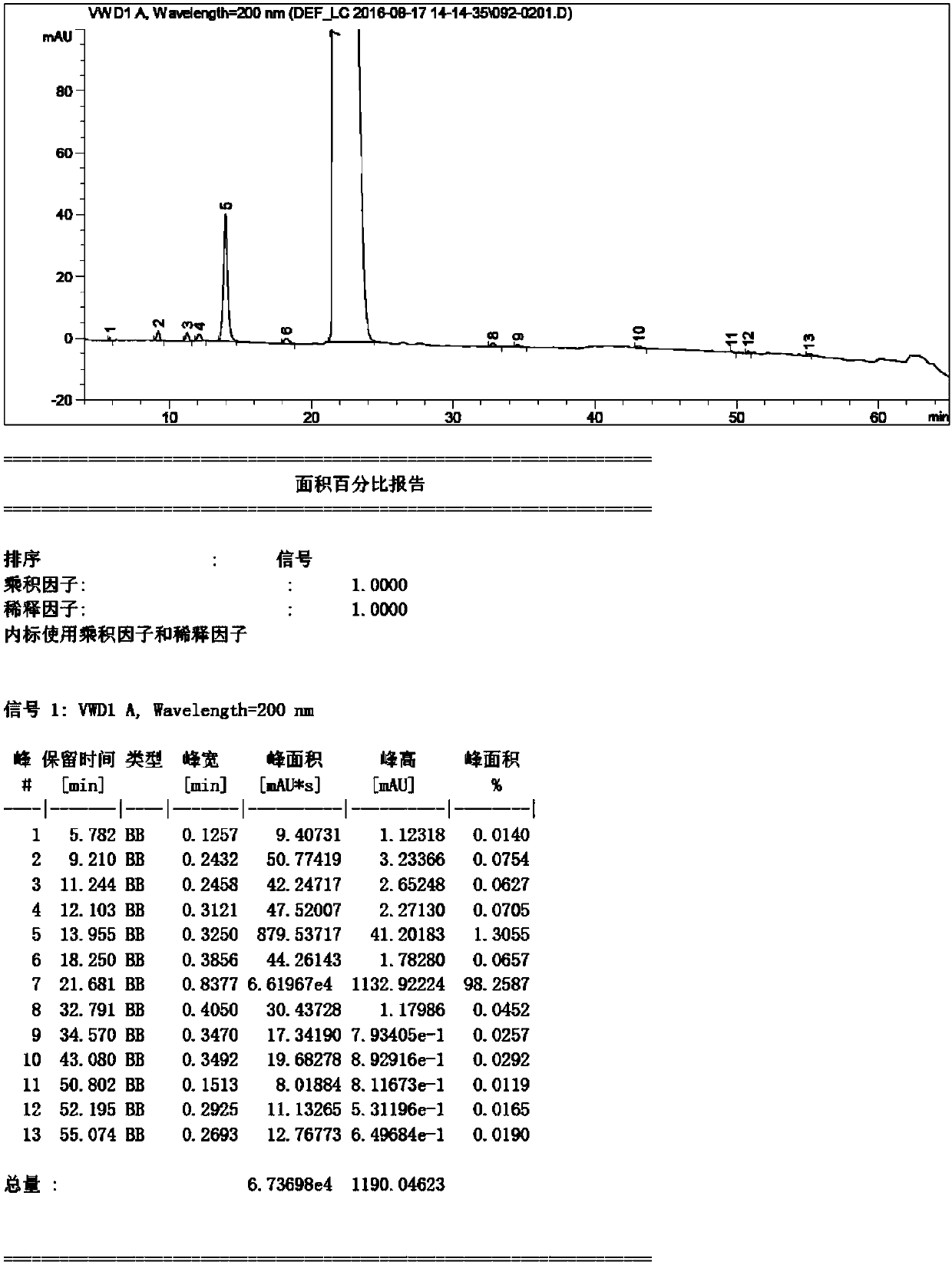
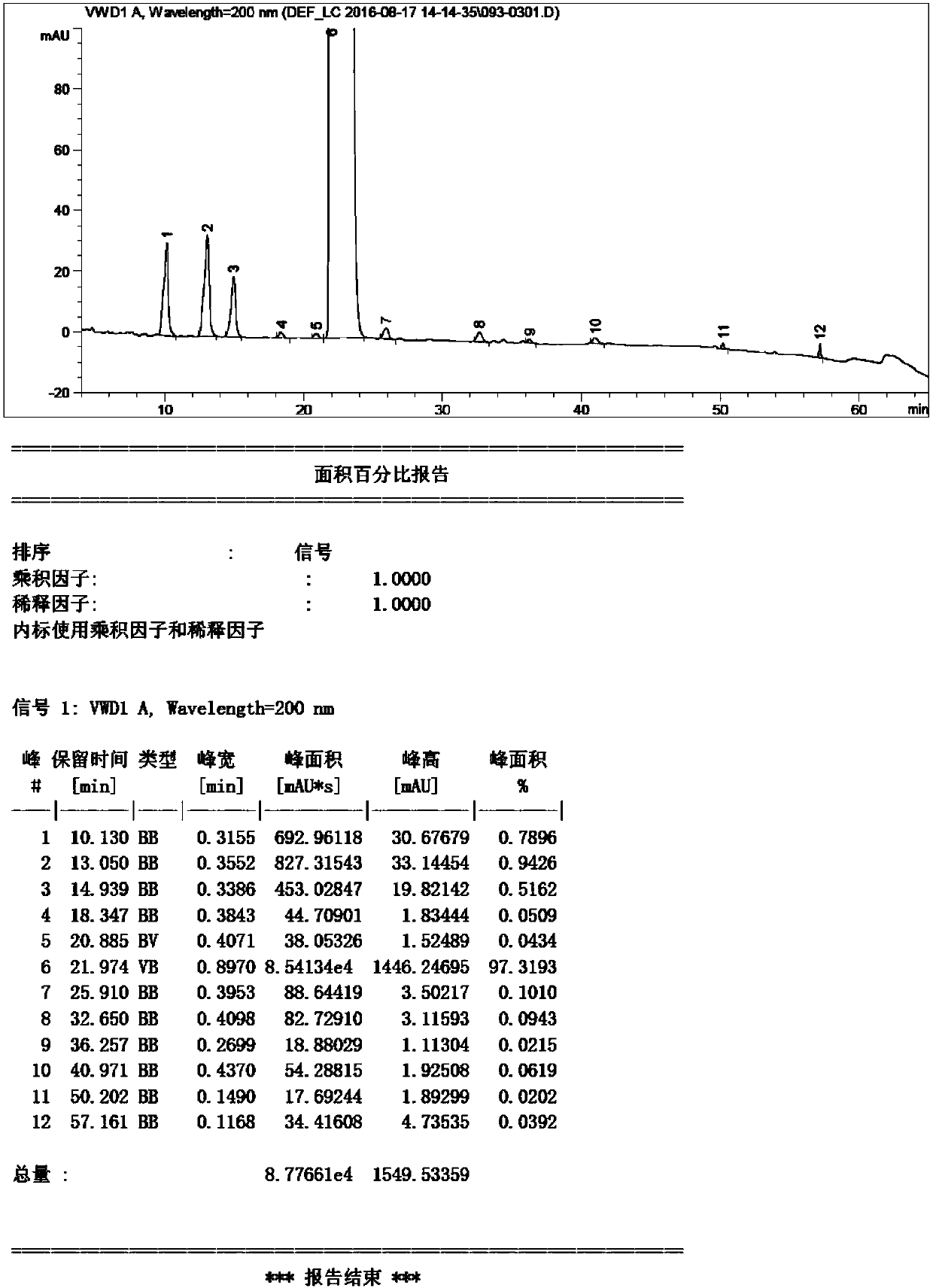
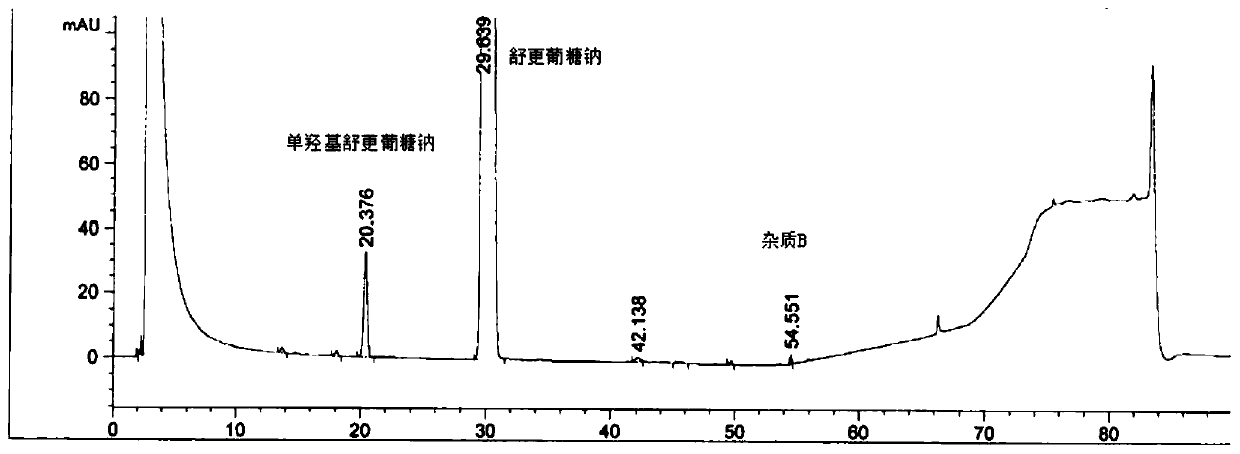
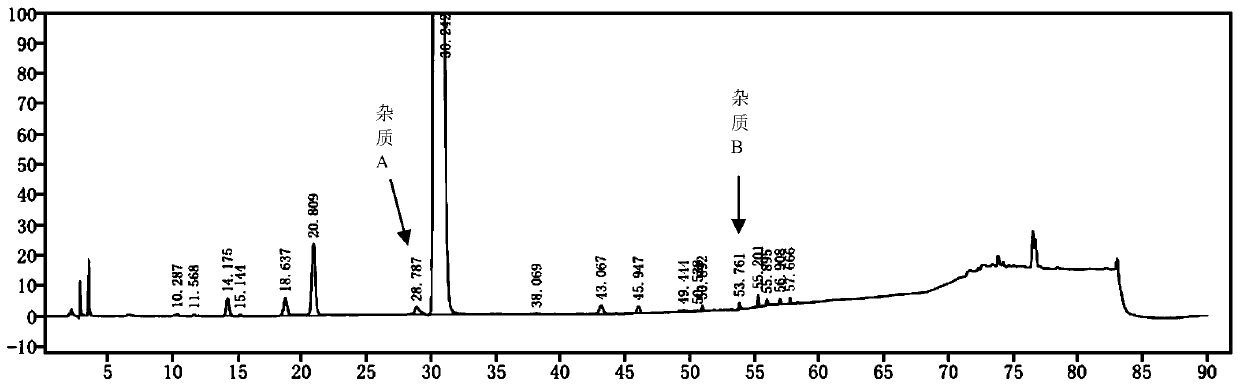
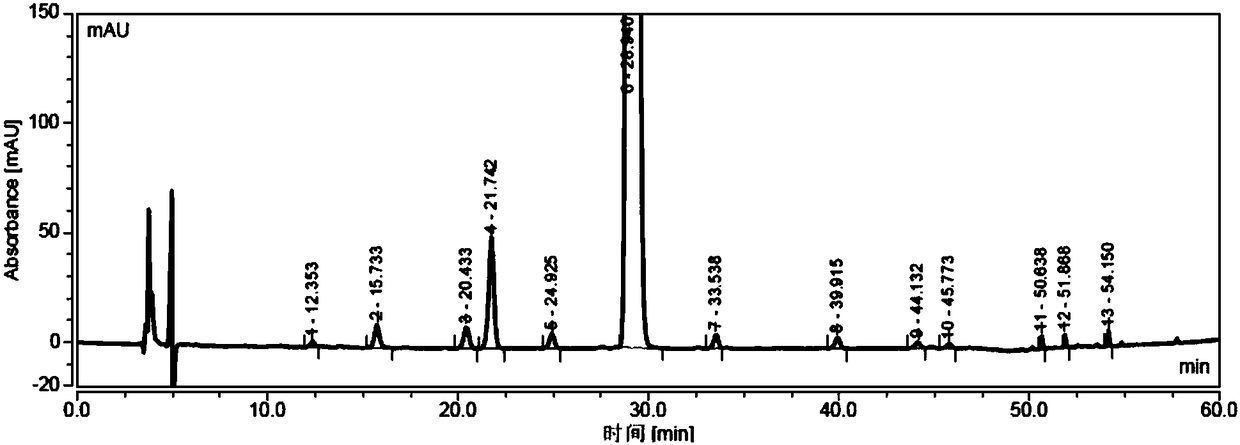
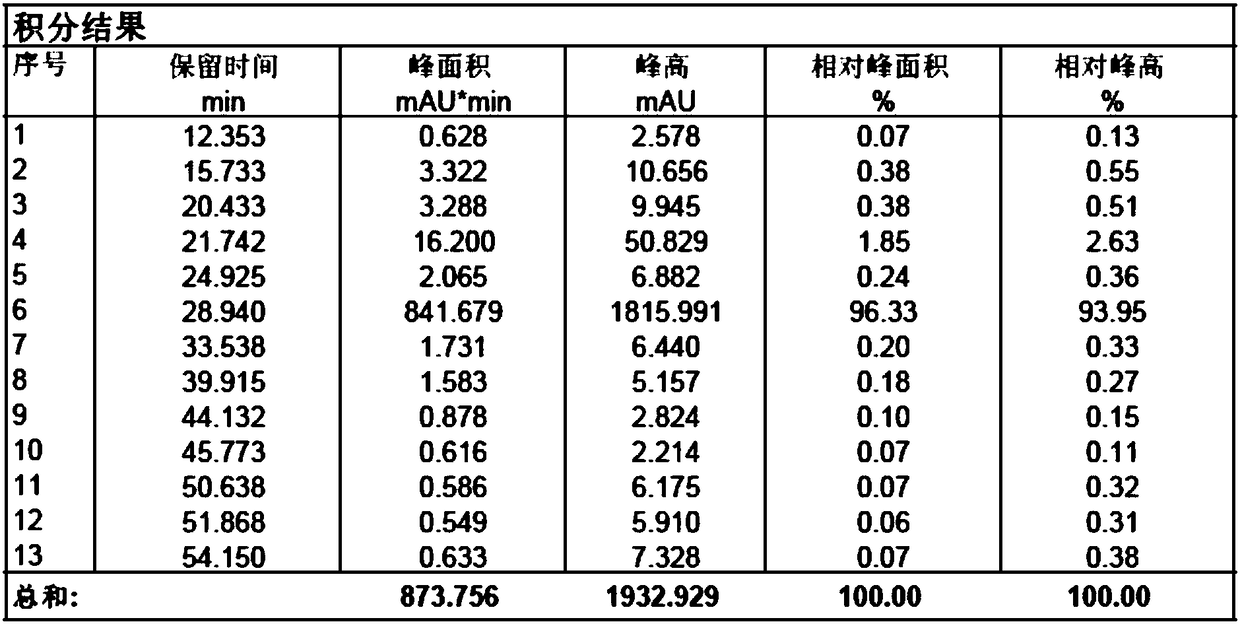
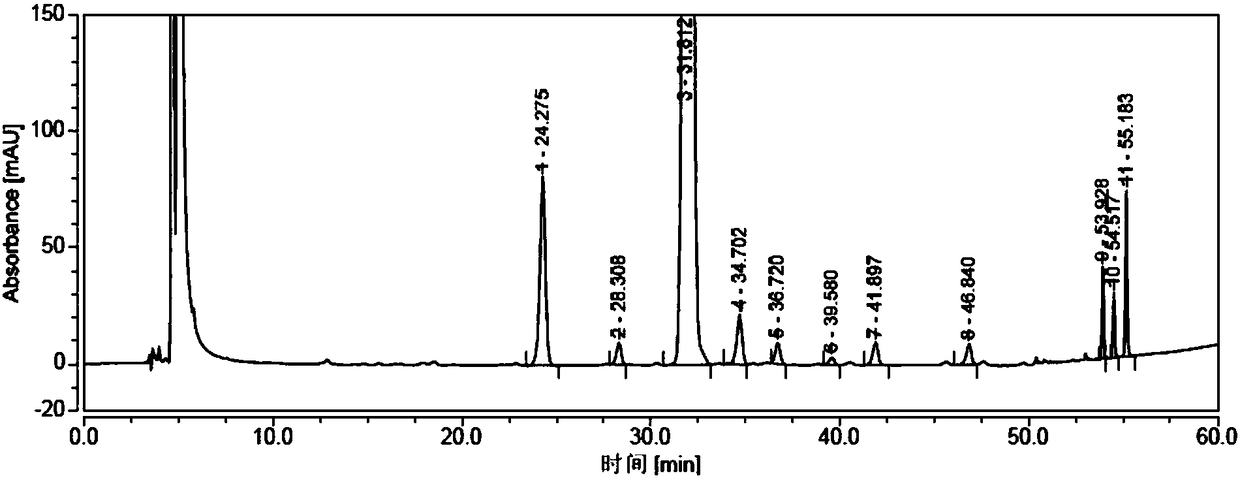
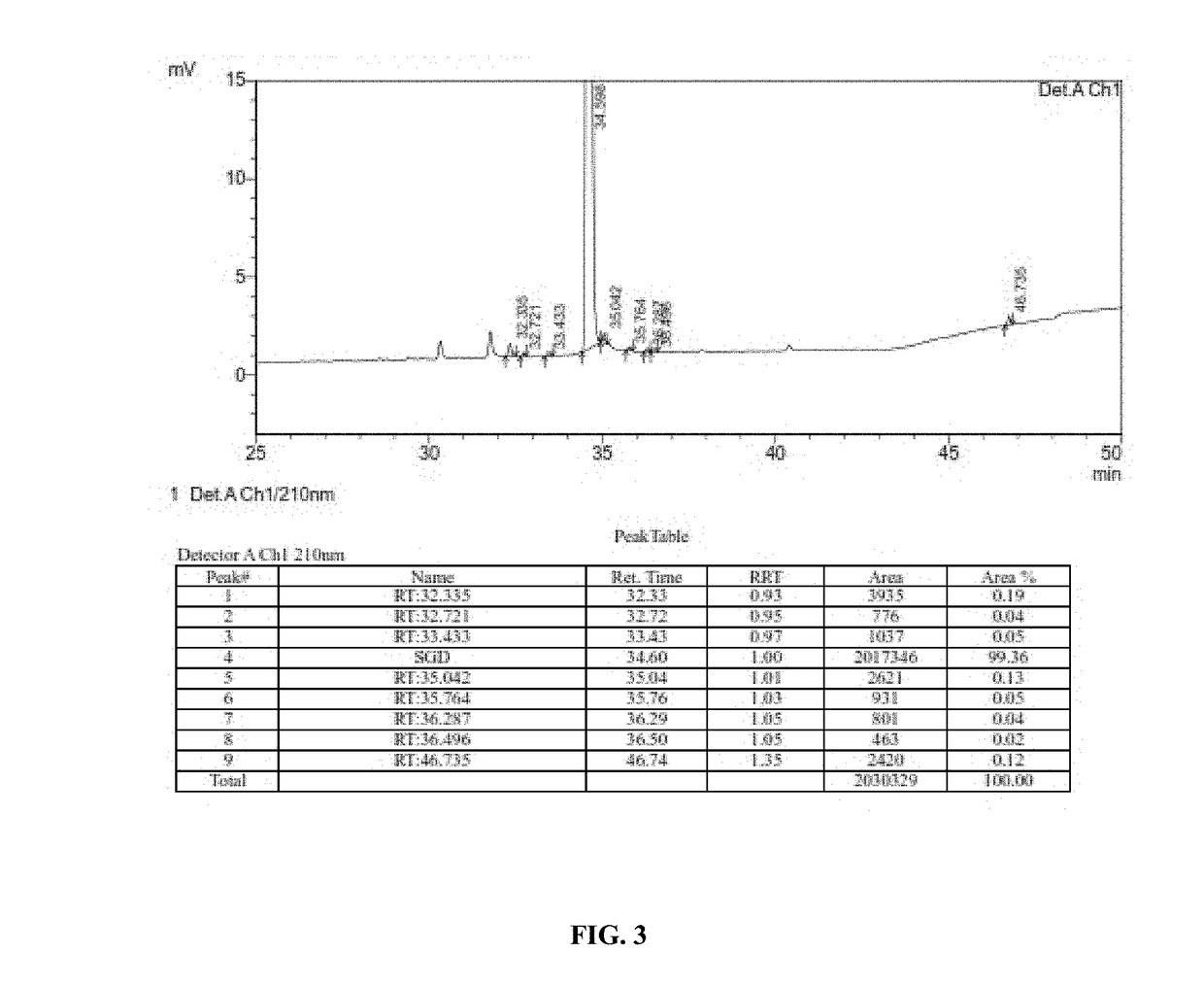
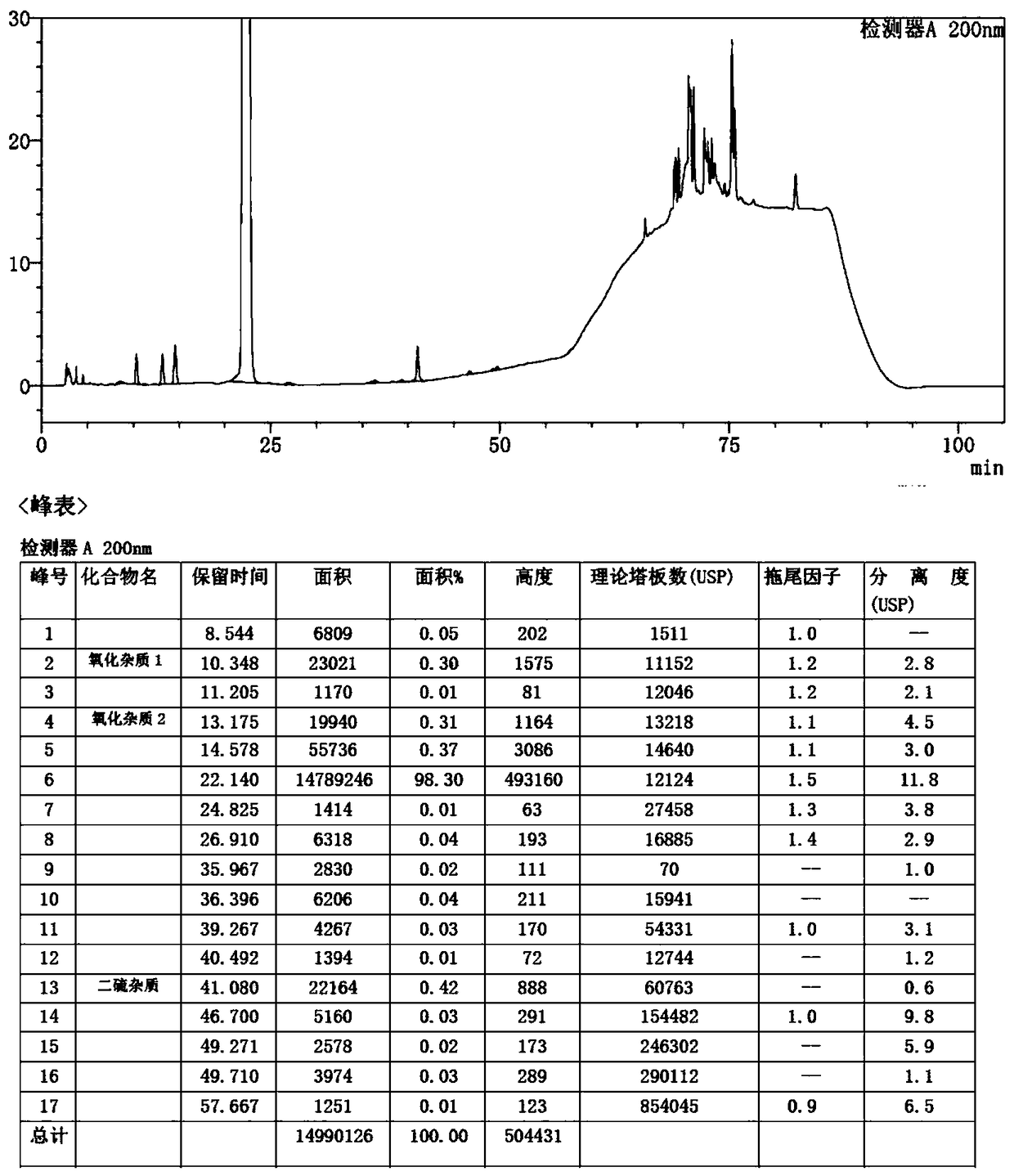
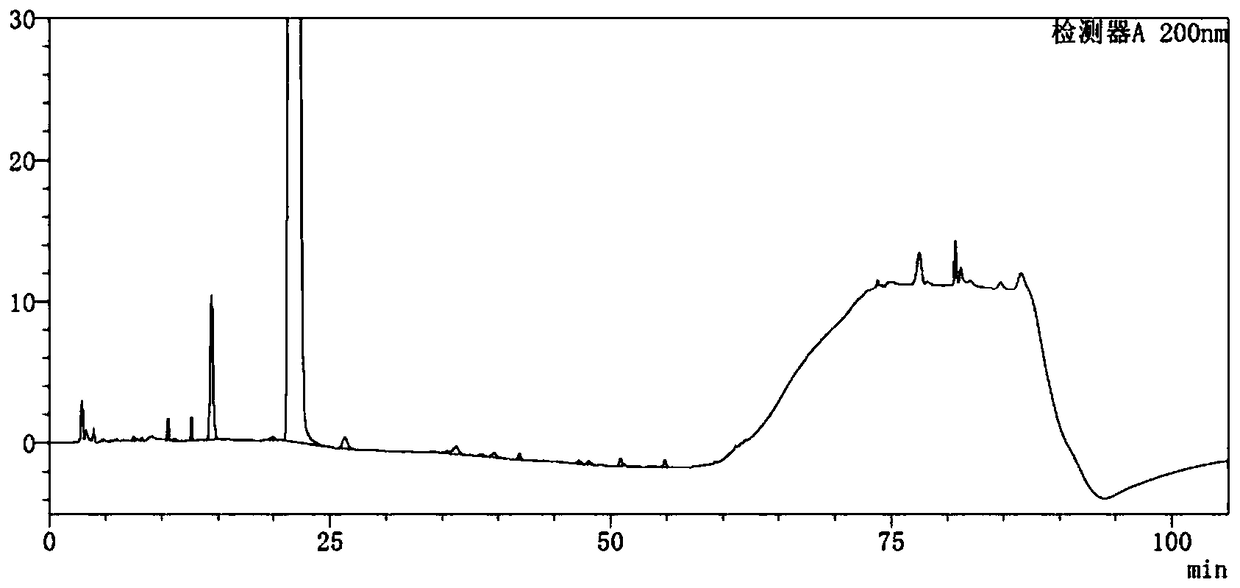
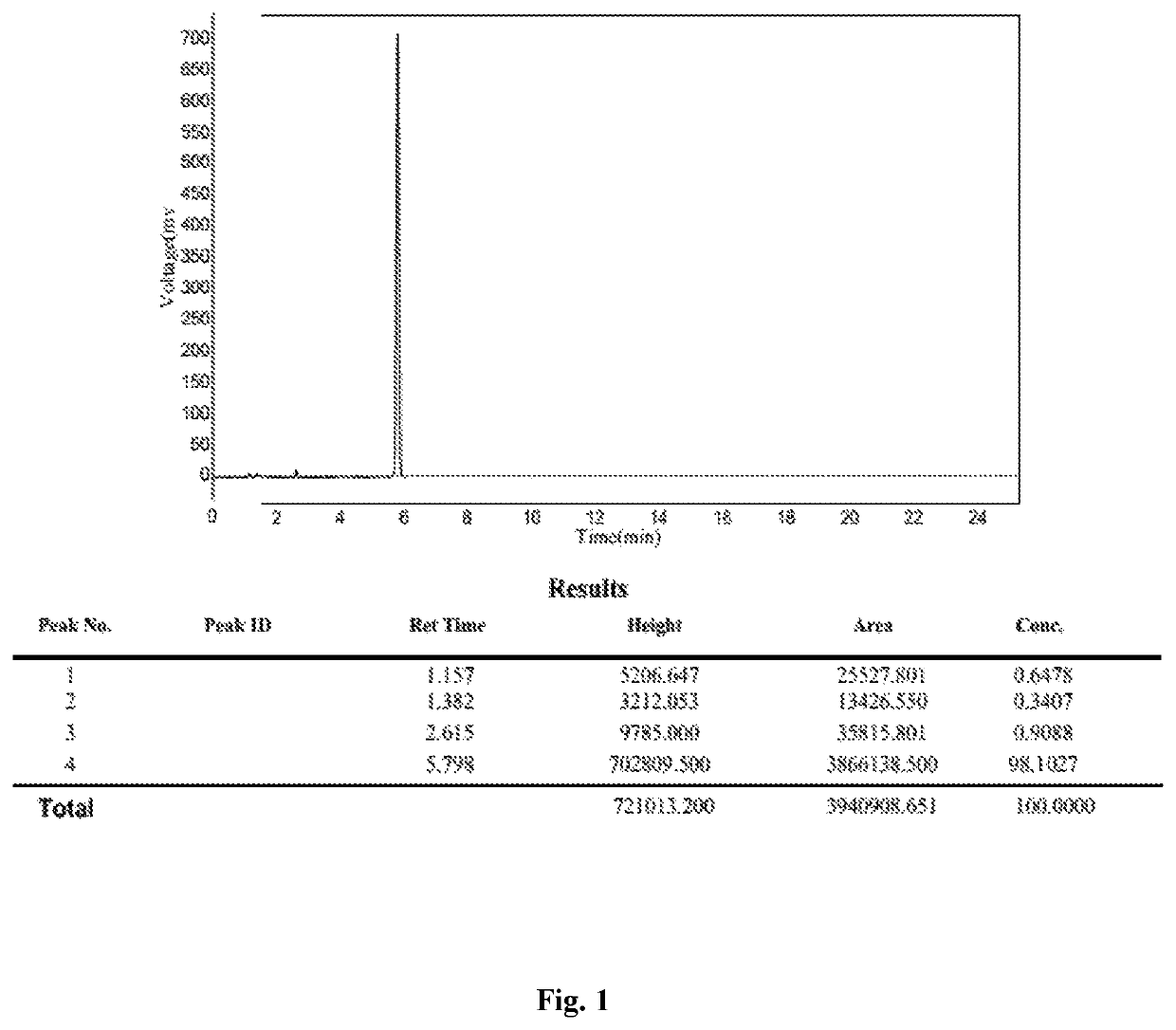
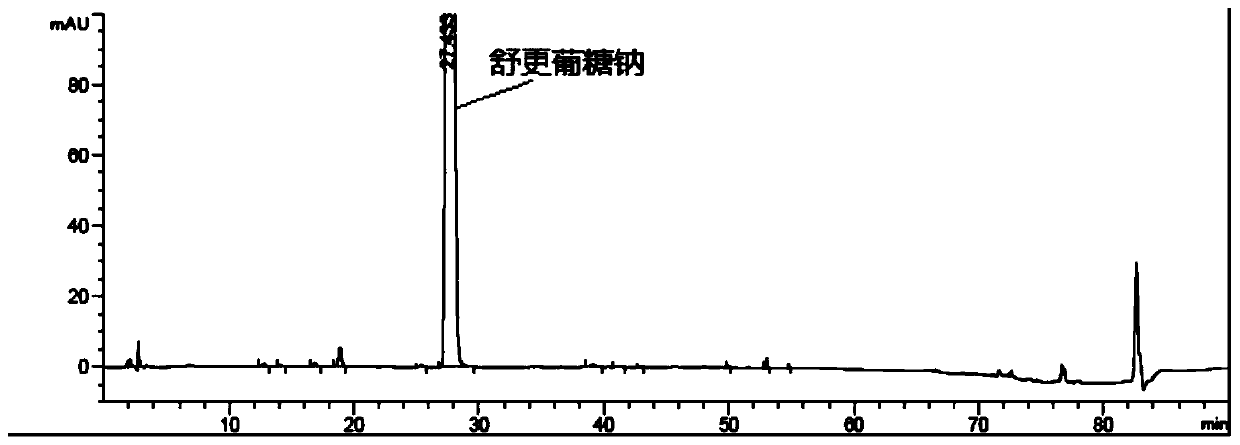
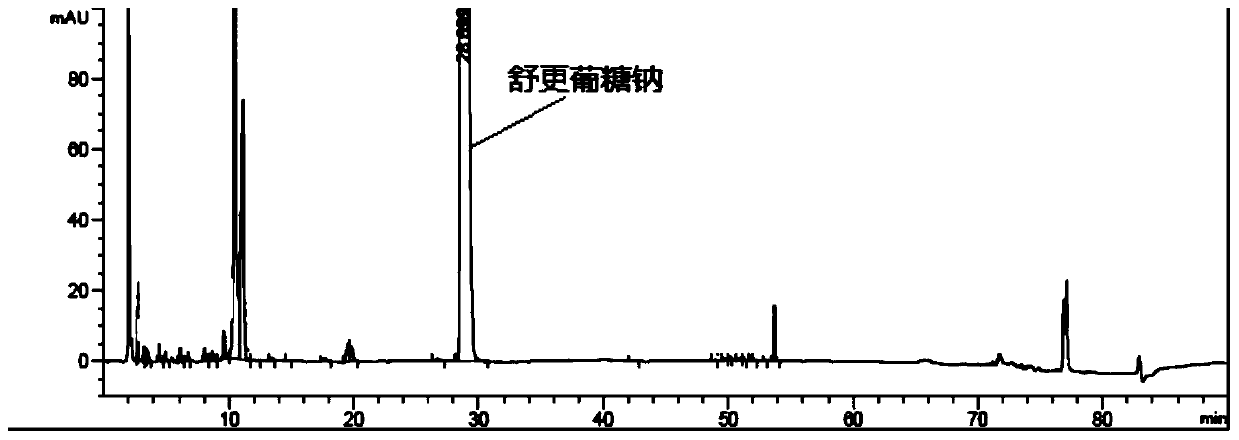
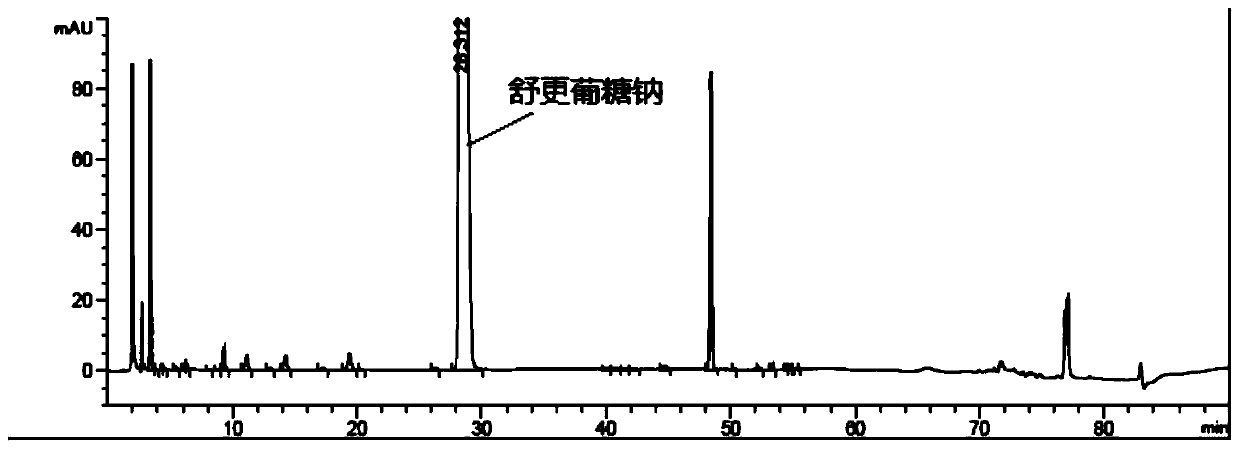
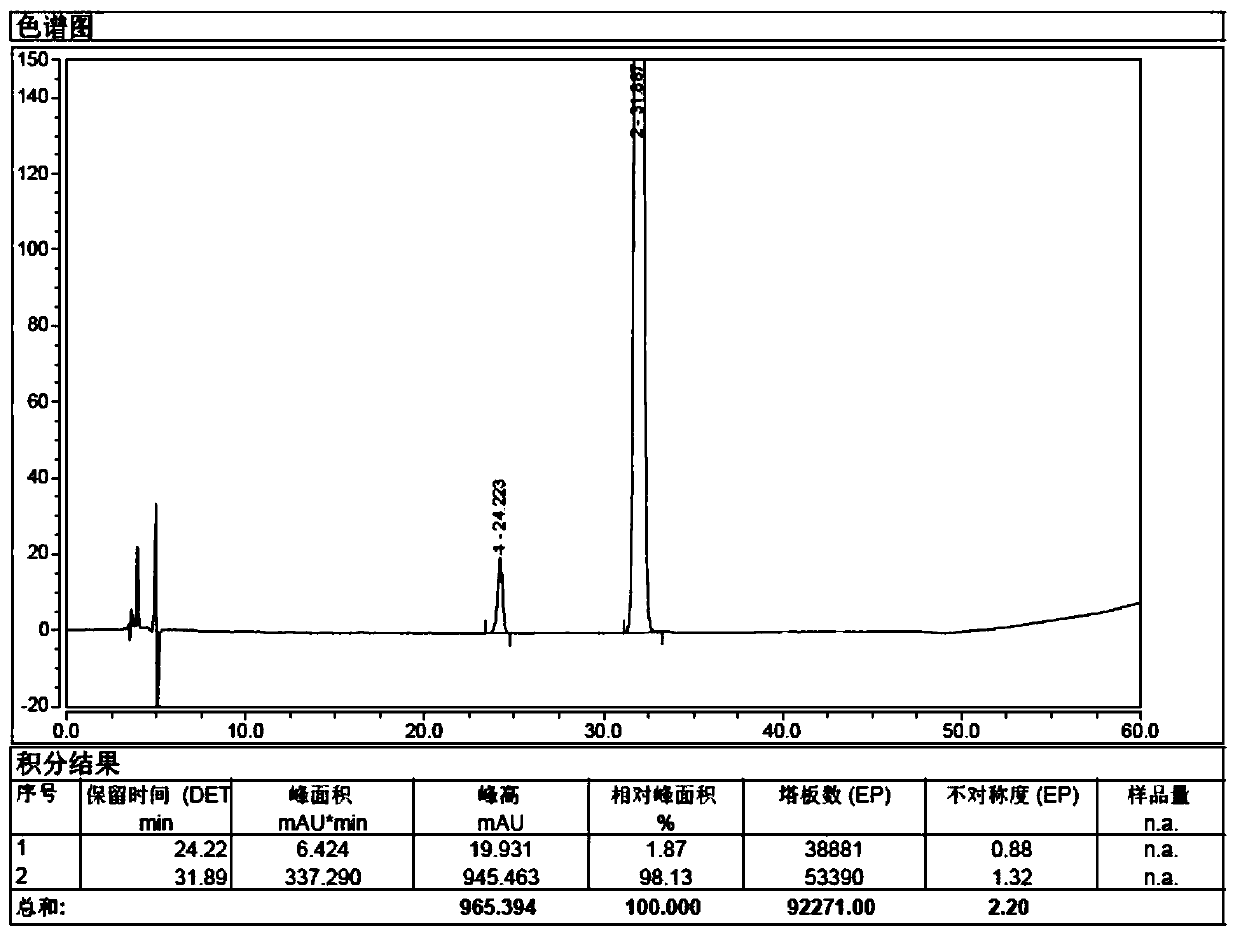
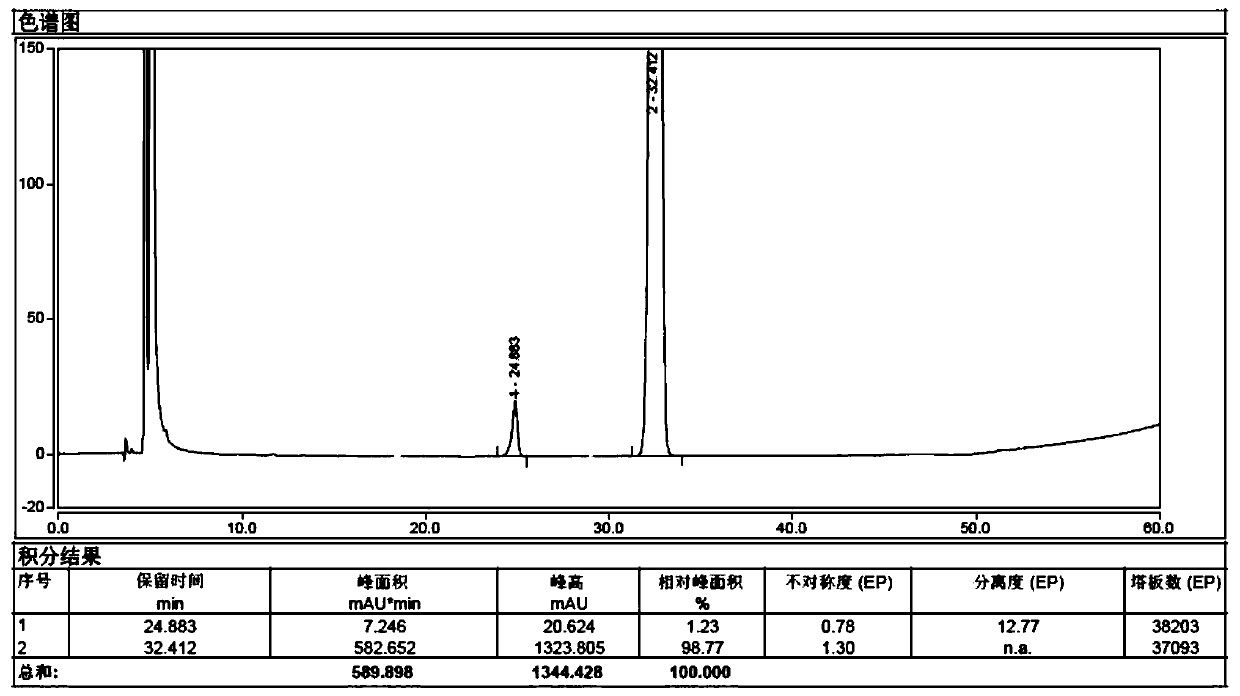
Detection method of sugammadex sodium
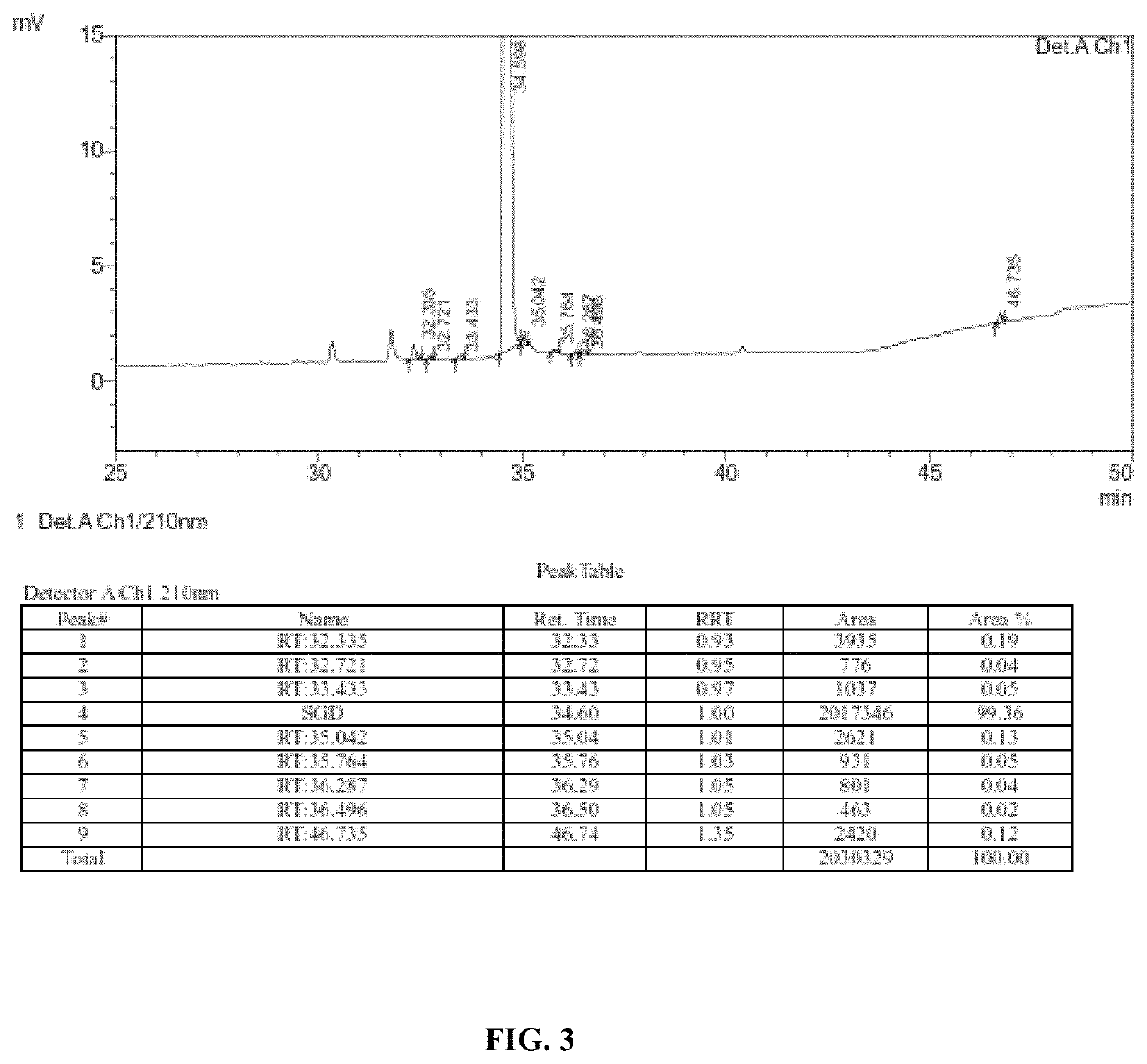
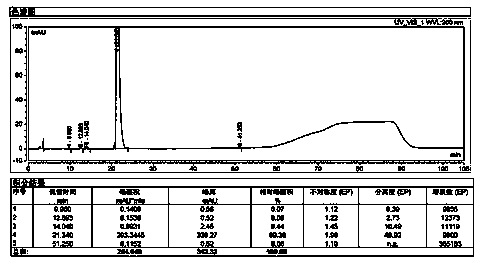
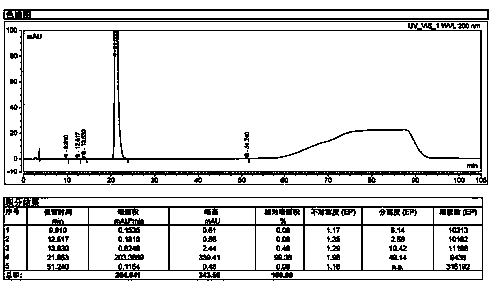
The invention relates to a method for detecting sugammadex sodium, which comprises the following steps of: respectively detecting a test sample solution and a reference substance solution by using a high performance liquid chromatograph under the chromatographic conditions that: a chromatographic column is a C18 chromatographic column; a mobile phase A is a phosphate buffer solution with the concentration of 5 mmol / L to 40 mmol / L or a phosphoric acid aqueous solution with the volume concentration of 0.1 percent to 1.0 percent, and the pH value of the mobile phase A is 2.0 to 2.9; a mobile phase B is acetonitrile; the column temperature of the chromatographic column is 30-45 DEG C; the flow rate is 0.6 ml / min to 1.2 ml / min; and the wavelength of the ultraviolet detection is 190 nm to 210 nm. According to the detection method of sugammadex sodium provided by the invention, sugammadex sodium related substances can be effectively separated, the sensitivity is high, the specificity is good,and the accuracy is high.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Purification method for intermediate prepared by sugammadex sodium
The invention discloses a purification method for an intermediate gamma-ICD prepared by sugammadex sodium. The method comprises the following steps: (1) crystallizing gamma-ICD by using a DMF-aqueoussolvent system to remove impurities greater in polarity first; and (2) recrystallizing the gamma-ICD again by using a DMF-acetone solvent system to remove impurities smaller in polarity. The method ofpurifying gamma-ICD cancels various complex purification processes and can obtain gamma-ICD relatively high in purity through a simple crystallizing process. The inventory of the follow-up sugammadexsodium reaction is reduced, the yield is improved, the cost is lowered, the environmental pollution is reduced, and industrial production is facilitated.
Owner:BRIGHTGENE PHARMA
Refining and crystallization method of sugammadex sodium
ActiveCN110818816AImprove crystal morphologyIncrease dissolution rateSugammadex SodiumPharmaceutical formulation
The invention relates to a refining and crystallization method of sugammadex sodium, in particular to a method for refining sugammadex sodium by dropwise adding an anti-solvent in portions, and belongs to the field of medicinal chemistry. The method has the advantages of simple process, good repeatability and stability, low environmental pollution, being suitable for industrial production, and relatively high product yield and purity. In addition, the dissolution rate of sugammadex sodium obtained by the method is also greatly improved, and thereby the high-quality bulk drug for the pharmaceutical preparation process is provided.
Owner:RUYUAN HEC PHARM
Preparation method of high-purity sugammadex sodium
ActiveCN111978435AReduce the risk of oxidationSuppress generationOrganic active ingredientsMuscular disorderInositol pentaphosphateSugammadex Sodium
The invention discloses a preparation method of high-purity sugammadex sodium. The high-purity sugammadex sodium is prepared from inositol phosphate and derivatives thereof. The specific process comprises the following steps of adding a specific type of protective agent into a crude sugammadex sodium product, and performing recrystallizing under the protection of inert gas to obtain a pure sugammadex sodium product, wherein the protective agent is selected from the inositol phosphate and the derivatives thereof, such as inositol hexaphosphate and salts or esters thereof, partial degradation products of the inositol hexaphosphate, such as inositol pentaphosphate, inositol tetraphosphate, inositol triphosphate, inositol diphosphate and inositol monophosphate, and one or a mixture of two or more of the above-mentioned substances and salts or esters thereof in any proportion. The method is simple and convenient to operate, high in product purity, good in safety, few in anaphylactic reaction, good in economical efficiency and more suitable for industrial production.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
A kind of purification method of sugammadex sodium
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Sugammadex sodium freeze-dried powder injection and preparation method thereof
PendingCN113456598ALess impuritiesSolve the problem of easy generation of large amounts of impuritiesPowder deliveryOrganic active ingredientsFreeze-dryingMedicine
The invention relates to the technical field of medicine technology, in particular to sugammadex sodium freeze-dried powder injection and a preparation method thereof. The preparation method of the sugammadex sodium freeze-dried powder injection comprises the following steps: adding sugammadex sodium into water for injection according to a prescription dosage, and stirring until sugammadex sodium is completely dissolved; adding a prescription amount of freeze-drying protection solution into the solution, stirring until the solution is completely dissolved, adjusting the pH value, stirring, filtering, filling and freeze-drying to obtain the target product. according to the method, the sugammadex sodium freeze-dried powder injection for injection is provided, and the problems that API serving as a cyclic molecular structure is unstable, and a large number of impurities are easily generated after high-temperature sterilization are solved; inert gas (nitrogen) is filled for protection after freeze-drying is finished, so that oxygen is effectively isolated, and the prepared sugammadex sodium freeze-dried powder injection has fewer impurities and is more stable; through optimization of a freeze-drying process, the prepared freeze-dried powder is shortest in redissolution time, the clarity and insoluble particles meet the requirements, the stability is higher, and the freeze-dried powder is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com