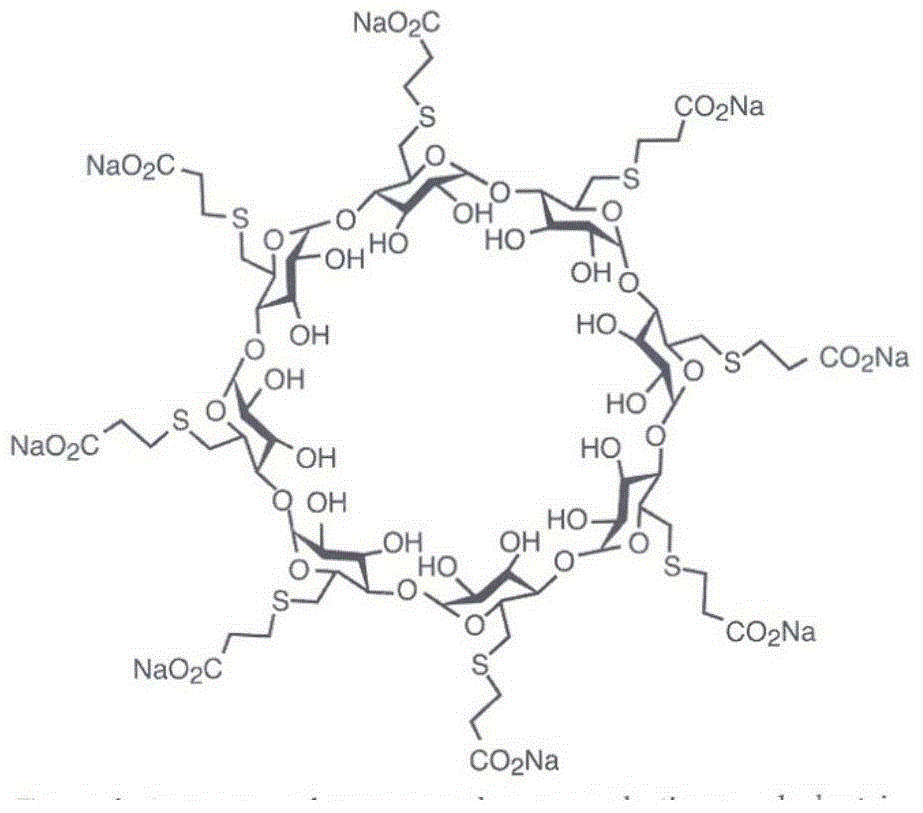
Method for purifying sugammadex sodium
A technique of sugammadex sodium and a purification method, which is applied in the field of drug synthesis, can solve problems such as low product purity and limited production capacity, and achieve the effects of increasing yield and purity and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
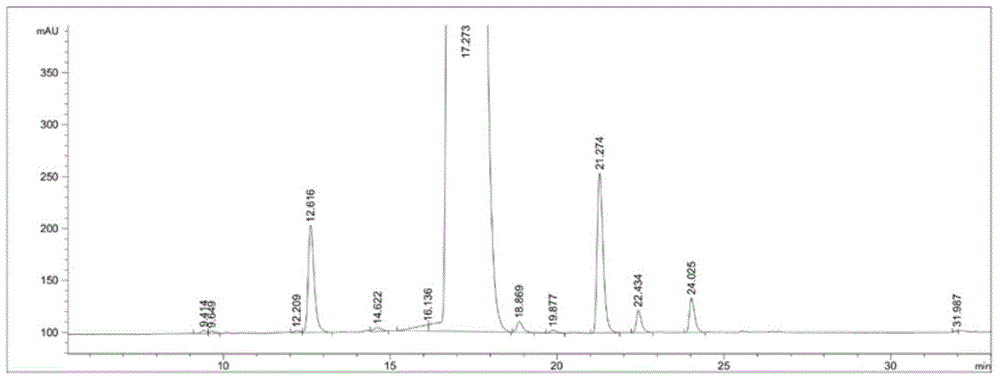
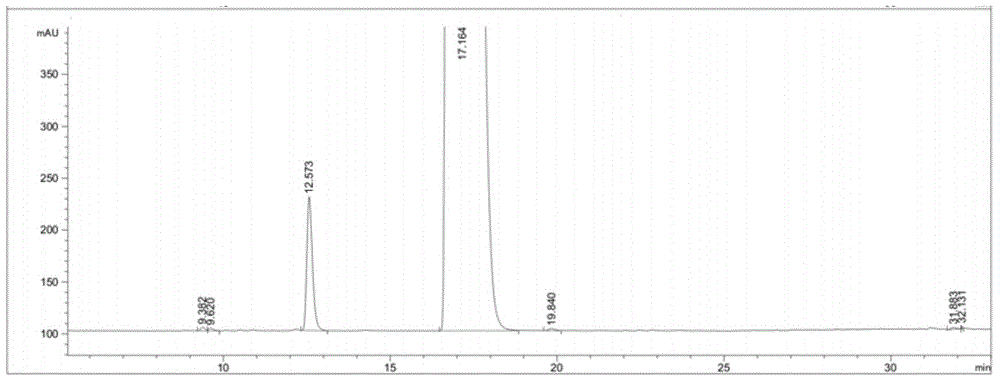
[0025] Embodiment 1: Purification of sugammadex sodium
[0026] Take 45 g of crude sugammadex sodium, dissolve it in 250 ml of water, adjust the pH value to 3 with 10% dilute hydrochloric acid, and precipitate a solid, beating and washing with water twice, suction filtration, and drying to obtain 36 g of sugammadex as a white solid.
[0027] Take 36g of the white solid prepared in the previous step and stir with 0.95g of ammonia water to obtain a clear light yellow solution. Add 1000ml of ethanol to precipitate a white solid, filter it with suction, and dry it in vacuo to obtain 30g of the white solid. Dilute the white solid with ethylene glycol / water Crystallized to a white solid, and dried to give sugammadex ammonium salt as a white solid.
[0028] Dissolve the dried sugammadex ammonium salt obtained in the previous step with 250ml of water, adjust the pH value to 3 with 10% dilute hydrochloric acid, precipitate a solid, wash twice with water, filter with suction, and dry to...
Embodiment 2
[0029] Embodiment 2: Purification of sugammadex sodium
[0030] Take 45g of sugammadex sodium crude product, dissolve it in 250ml of water, adjust the pH value to 3 with 20% dilute hydrochloric acid, and precipitate a solid, beating and washing with water twice, suction filtration, and drying to obtain 36g of white solid sugammadex
[0031] Take 36g of the white solid prepared in the previous step, feed ammonia gas, stir and react to obtain a clear light yellow solution, add 1000ml of ethanol, precipitate a white solid, filter with suction, and dry in vacuo to obtain 30g of a white solid. Recrystallize from water to obtain white solid, and dry to obtain sugammadex ammonium salt as white solid.
[0032] Dissolve the dried sugammadex ammonium salt obtained in the previous step with 250ml of water, adjust the pH value to 3 with 20% dilute hydrochloric acid, precipitate a solid, wash twice with water, filter with suction, and dry to obtain sugammadex as a white solid Carboxylic a...
Embodiment 3
[0033] Embodiment 3: Purification of sugammadex sodium
[0034] Take 45g of sugammadex sodium crude product, dissolve it in 250ml of water, adjust the pH value to 5 with 5% dilute hydrochloric acid, and precipitate a solid, beating and washing with water twice, suction filtration, and drying to obtain 36g of white solid sugammadex
[0035] Take 36 g of the white solid sugammadex prepared in the previous step and stir with 0.63 ammonia water to obtain a clear light yellow solution. Add 1000 ml of ethanol to precipitate a white solid, filter it with suction, and dry it in vacuum to obtain 30 g of the white solid. Alcohol / water recrystallization to give white solid, drying to give sugammadex ammonium salt as white solid.
[0036] Dissolve the dried sugammadex ammonium salt obtained in the previous step with 250ml of water, adjust the pH value to 5 with 5% dilute hydrochloric acid, precipitate a solid, wash twice with water, filter with suction, and dry to obtain sugammadex as a w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com