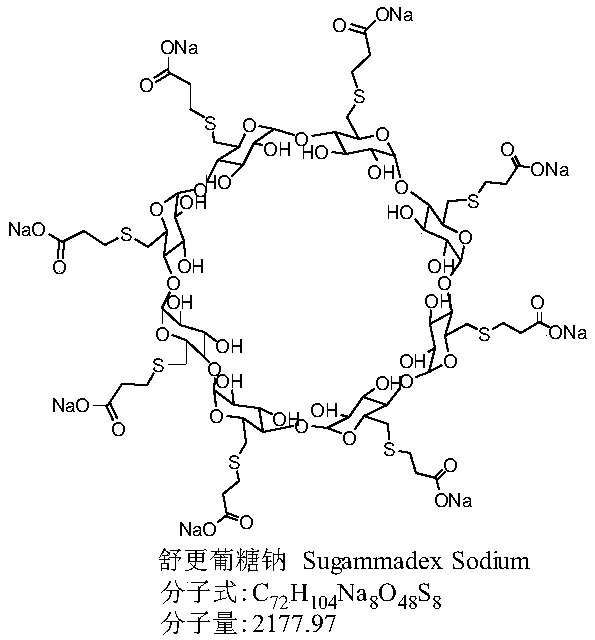
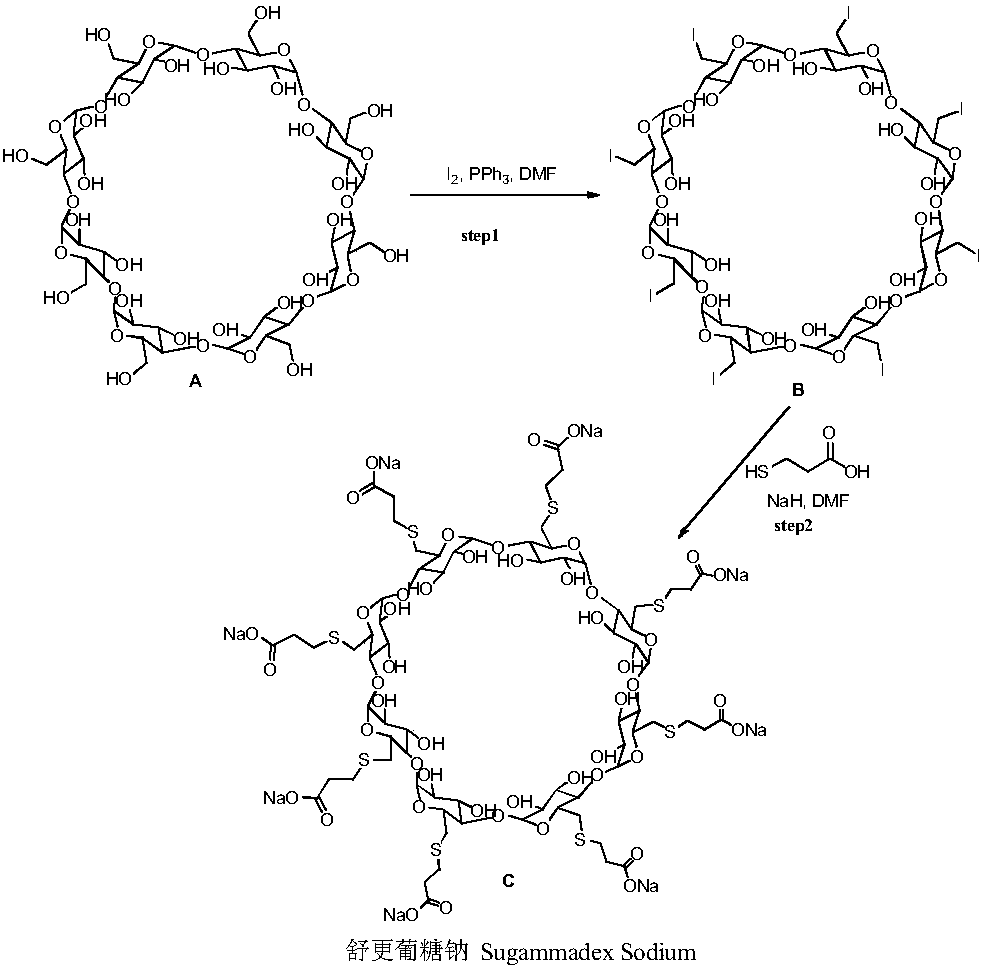
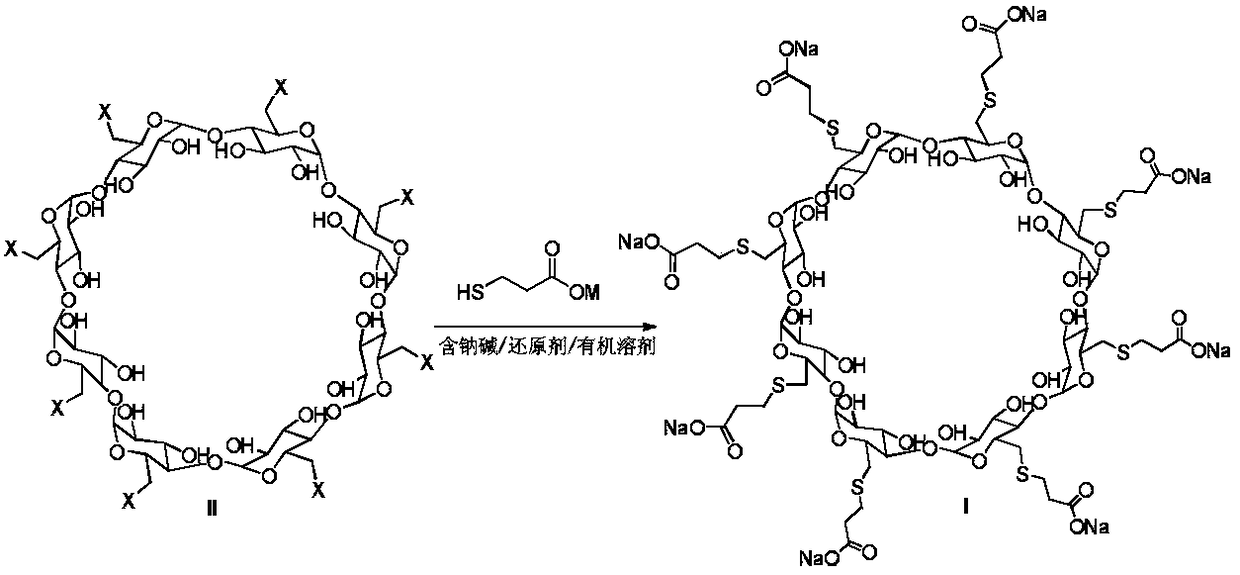
Method for preparing sugammadex sodium
A technology of sugammadex sodium and sodium base, applied in the field of drug synthesis, can solve the problems of low yield, difficult to purify, not much, easy to be oxidized, etc., and achieve the effect of improving the total yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Sodium hydride (706 g, 60%) was added to dry DMF (31.2 L) under nitrogen and ice bath. Slowly add the mixed solution of tri-p-tolylphosphine (172g)-3-mercaptopropionic acid (938g)-DMF (1.2L) dropwise at 0~10°C. Add all-iodide γ-cyclodextrin (1.2kg)-tri-p-tolylphosphine (45g)-DMF (6.25L) mixed solution, and continue stirring for about 4 hours. The reaction solution was lowered to 0-10°C, water (6 L) was added, and the temperature was raised to 55-70°C with stirring for about 2 hours. Cool the reaction solution to room temperature, filter with suction, dissolve the filter cake with water (9.6L), filter with diatomaceous earth, add ethanol (24L) to the filtrate, and filter with suction to obtain the crude product of sugammadex sodium (1.1kg, harvested Yield 92%, purity 91.35%).
Embodiment 2
[0073]Example 2: Sodium hydride (58.8 g, 60%) was added to dry DMF (2.6 L) under nitrogen protection and ice bath. Slowly add the mixed solution of triphenylphosphine (12.3g)-3-mercaptopropionic acid (78.2g)-DMF (0.5L) dropwise at 0-10°C, heat up to 65-75°C and react under stirring, then slowly Add the mixed solution of periodo γ-cyclodextrin (100g)-triphenylphosphine (3.9g)-DMF (0.7L) dropwise, and continue stirring for about 6h. The reaction solution was lowered to 0-10°C, water (0.6 L) was added, and the temperature was raised to 55-70°C for about 3 hours under stirring. Cool the reaction solution to room temperature, filter with suction, dissolve the filter cake with water (1.0 L), filter with diatomaceous earth, add ethanol (2.0 L) to the filtrate, and filter with suction to obtain the crude sugammadex sodium (90 g, harvested Yield 90%, purity 90.97%).
Embodiment 3
[0074] Example 3: Sodium methoxide (79.6 g) was added to dry DMI (2.6 L) under nitrogen protection and ice bath. Slowly add the mixed solution of tricyclohexylphosphine (29.0g)-3-mercaptopropionic acid (78.2g)-DMI (0.5L) dropwise at 0-10°C, heat up to 65-75°C and react under stirring, then slowly Add the mixed solution of all-iodide γ-cyclodextrin (100g)-tricyclohexylphosphine (9.0g)-DMI (0.7L) dropwise, and continue stirring for about 8 hours. The reaction solution was lowered to 0-10°C, water (0.6 L) was added, and the temperature was raised to 55-70°C for about 4 hours under stirring. Cool the reaction solution to room temperature, filter with suction, dissolve the filter cake with water (1.0 L), filter with diatomaceous earth, add ethanol (2.0 L) to the filtrate, and filter with suction to obtain the crude sugammadex sodium (90 g, harvested 90% yield, 89.35% purity)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com