Sugammadex sodium impurity and preparing method thereof
A technology of sugammadex sodium and impurities, applied in the field of drug synthesis, can solve the problem of not clearly obtaining sugammadex sodium oxide impurities, etc., and achieve the effects of high purity, high yield and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Reaction formula:
[0037]
[0038] Take sugammadex sodium 2.0g, add it to 5ml water, stir to dissolve and clarify, then add 20mg 30% H 2 o 2 , after stirring at 50° C. for 3 h, cooled to room temperature, and then added 10 ml of methanol, precipitated solid, filtered, and dried to obtain 2.1 g of solid, compound II: 16.1%, compound III: 20.4%. Then, the crude oxide was prepared and separated to obtain 70 mg of compound II with a purity of 92%, and 62 mg of compound III with a purity of 96%.
[0039] Compound I:
[0040] MS(m / z):1006.5[M-8Na+6H] - / 2.
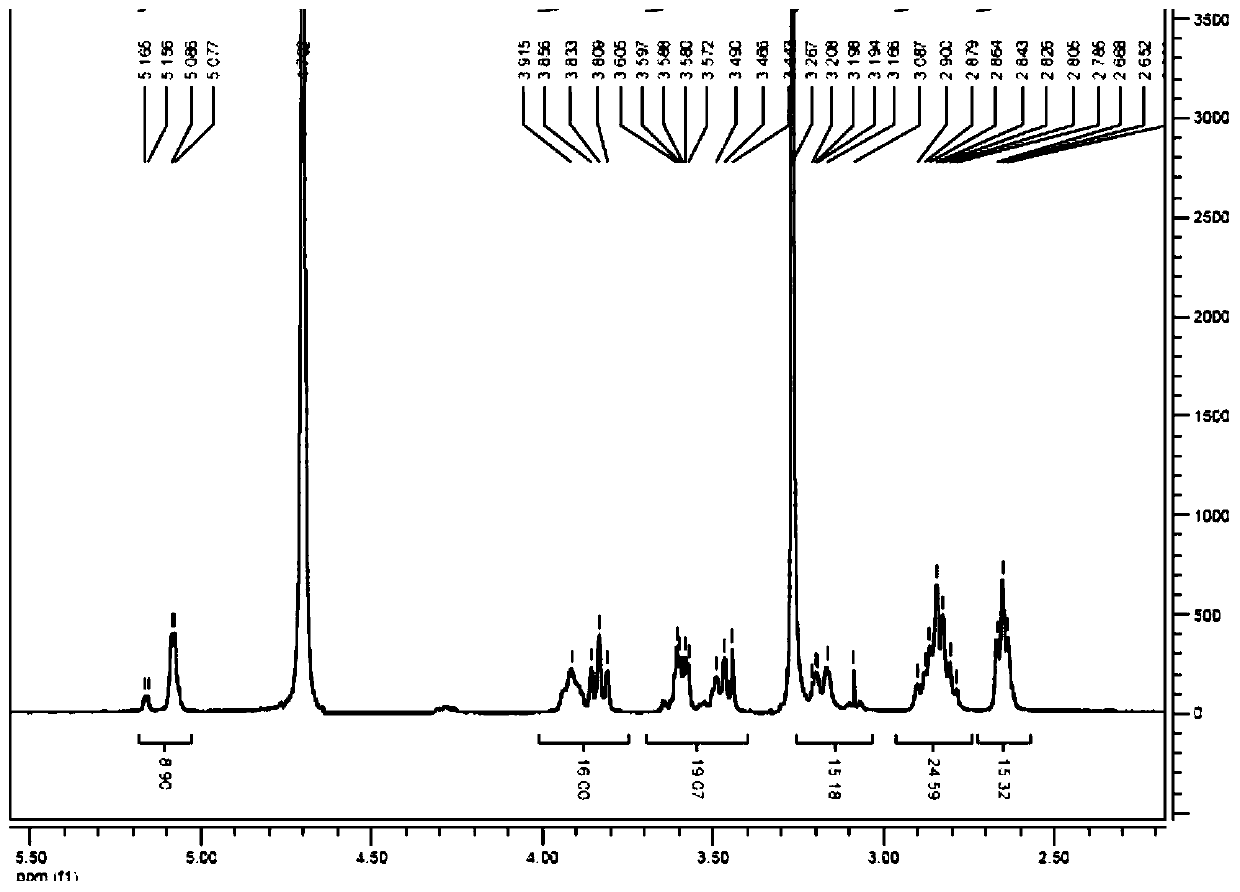
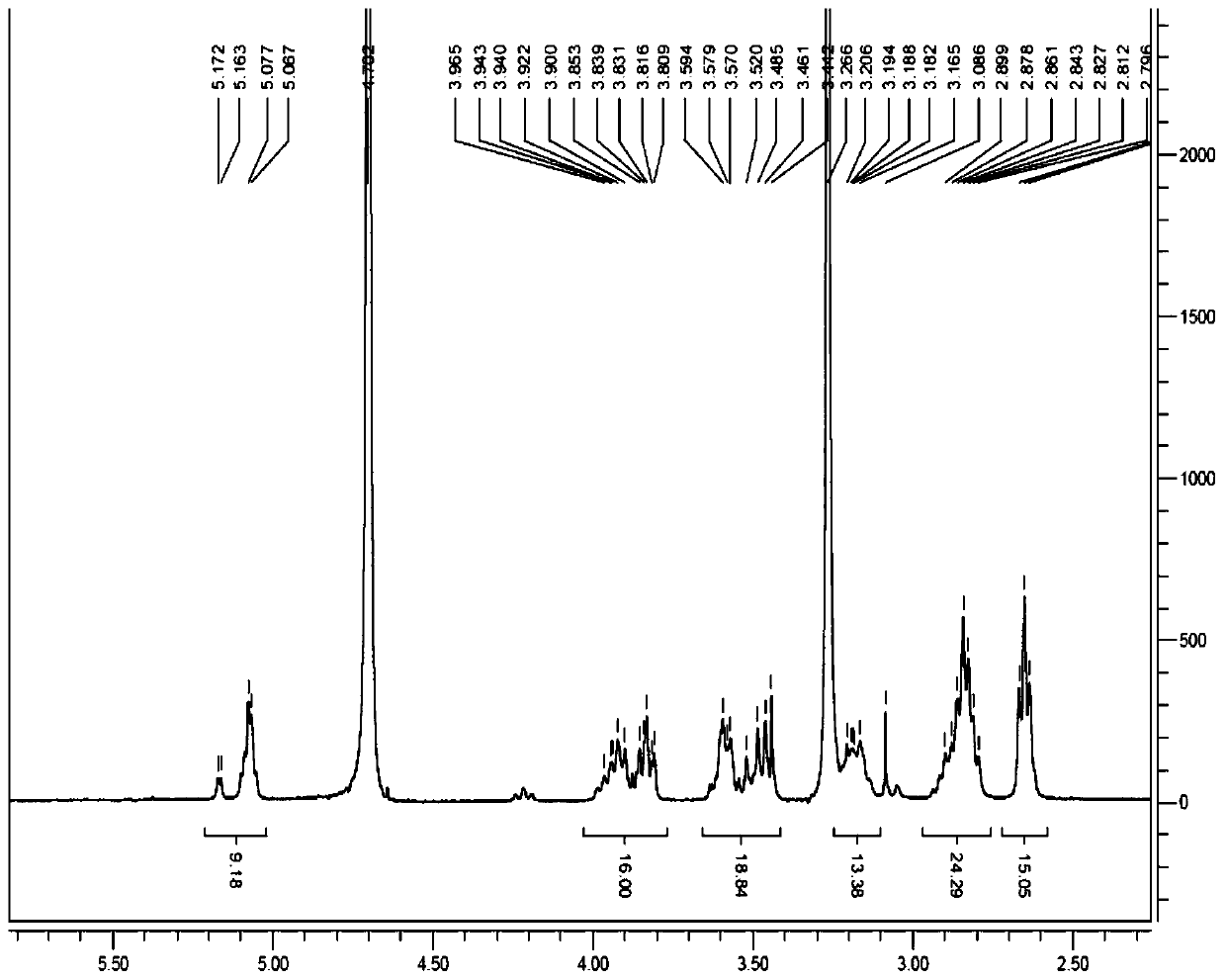
[0041] 1 H-NMR (400MHz,D 2 O): δ2.63~2.66(m,16H), 2.78~2.90(m,24H), 3.10~3.20(m,8H), 3.44~3.60(m,16H), 3.80~3.91(m,16H), 5.07~5.16(m,8H).
[0042] Compound II:
[0043] MS(m / z):1006.9[M-8Na+6H] - / 2.
[0044] 1 H-NMR (400MHz,D 2 O): δ2.63~2.66(m,16H), 2.79~2.91(m,24H), 3.13~3.22(m,8H), 3.44~3.59(m,16H), 3.80~3.98(m,16H), 5.05~5.17(m,8H).
Embodiment 2
[0046] Reaction formula:
[0047]
[0048] Take 3.0g of sugammadex sodium, add it to 6ml of water, stir to dissolve and clarify, then add 30mg of 30% H 2 o 2 , after stirring at 50° C. for 3 h, cooled to room temperature, and then added 15 ml of methanol, precipitated solid, filtered, and dried to obtain 3.2 g of solid, compound II: 13.1%, compound III: 18.4%. Then, the crude oxide was prepared and separated to obtain 100 mg of compound II with a purity of 93%, and 70 mg of compound III with a purity of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com