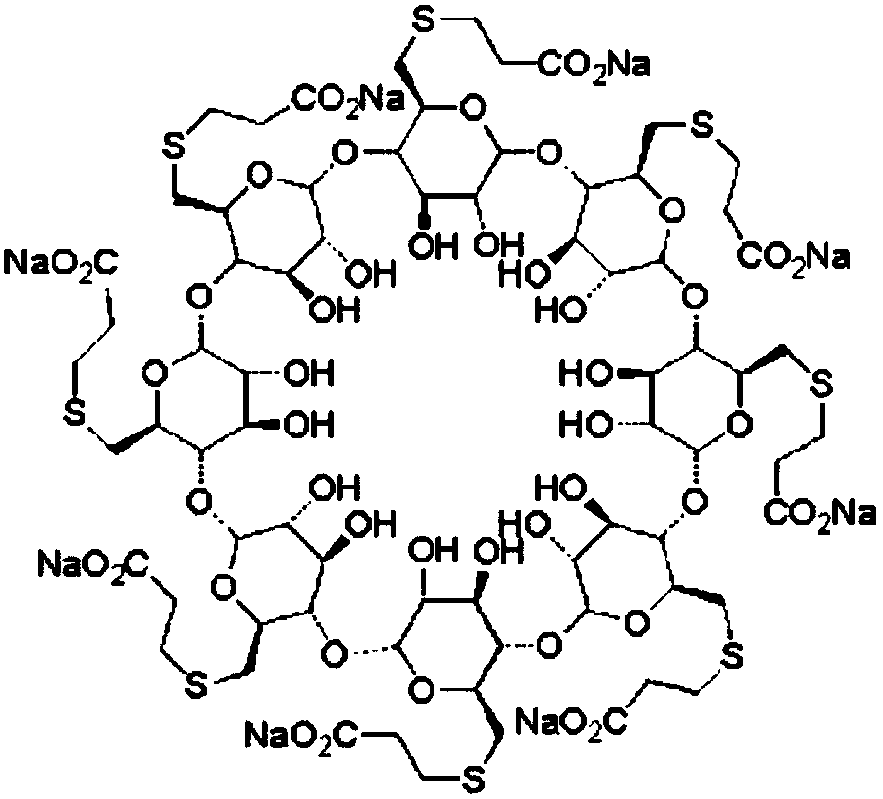
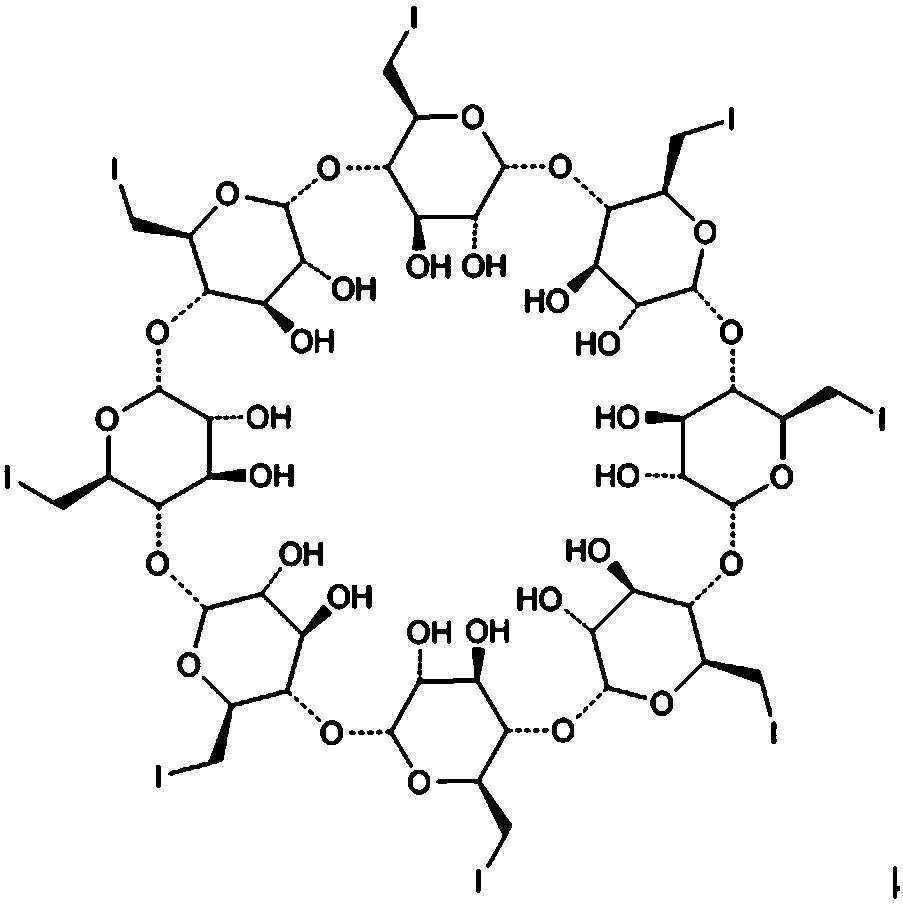
Purification method for intermediate prepared by sugammadex sodium
A purification method and crude product technology, which is applied in the field of pharmaceutical preparation, can solve problems such as poor purification effect, complex structure of γ-ICD, and difficult intermediates, and achieve the effects of industrial production, cost reduction, and yield improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of γ-ICD
[0018] In a 2000mL three-neck flask, under nitrogen protection, add 160ml of DMF and triphenylphosphine (30.1g, 15eq), and add iodine (30.5g, 15.6eq), exothermic violently, control the temperature at 25±5°C, and stir for 10min. Add dry good γ-cyclodextrin (10 g, 7.7 mmol), after the addition is complete, raise the temperature to 70° C. and stir for reaction for 24 hours. Cool the reaction solution to 10°C, add sodium methoxide solution (3.1g sodium added to 50ml methanol), stir for 30min, then add methanol 800ml, add 500ml purified water, and filter the solid. Crude γ-ICD 18.5 g was washed with water (3 x 100 ml) and then with acetone (3 x 100 ml).
Embodiment 2
[0020] Purification of γ-ICD
[0021] In a 500ml three-necked flask, under the protection of nitrogen, add 18.5g of γ-ICD obtained in Example 1, add 185g of DMF, raise the temperature to 70°C, add 129.5g of purified water dropwise, after completion of the dropwise addition, a large amount of solids are precipitated, and the temperature is naturally lowered slowly. Stir at 25°C for 4 hours, and filter to obtain a wet product of γ-ICD.
[0022] In a 500ml three-necked flask, under the protection of nitrogen, add the wet product of γ-ICD obtained in the previous step, add 40g of DMF, raise the temperature to 70°C, add 200g of acetone solution, cool down to 25°C, stir for 4h, filter the wet product by suction, and use vacuum drying , the water temperature was set at 70° C., and dried for 8 hours to obtain pure γ-ICD with a yield of 92% and a purity of 99.86%.
Embodiment 3
[0024] Purification of γ-ICD
[0025] In a 500ml three-necked flask, under the protection of nitrogen, add 18.5g of γ-ICD obtained in Example 1, add 92.5g of DMF, raise the temperature to 50°C, add 92.5g of purified water dropwise, after the completion of the dropwise addition, a large amount of solids are precipitated, and the temperature is naturally lowered slowly , stirred at 25°C for 4 hours, and filtered to obtain a wet product of γ-ICD.
[0026] In a 500ml three-necked flask, under the protection of nitrogen, add the wet product of γ-ICD obtained in the previous step, add 27.75g of DMF, heat up to 80°C, add 222g of acetone solution, cool down to 25°C, stir for 4h, filter the wet product, and use a vacuum After drying, the water temperature was set at 70° C. and dried for 8 hours to obtain a pure γ-ICD product with a yield of 85% and a purity of 97.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com