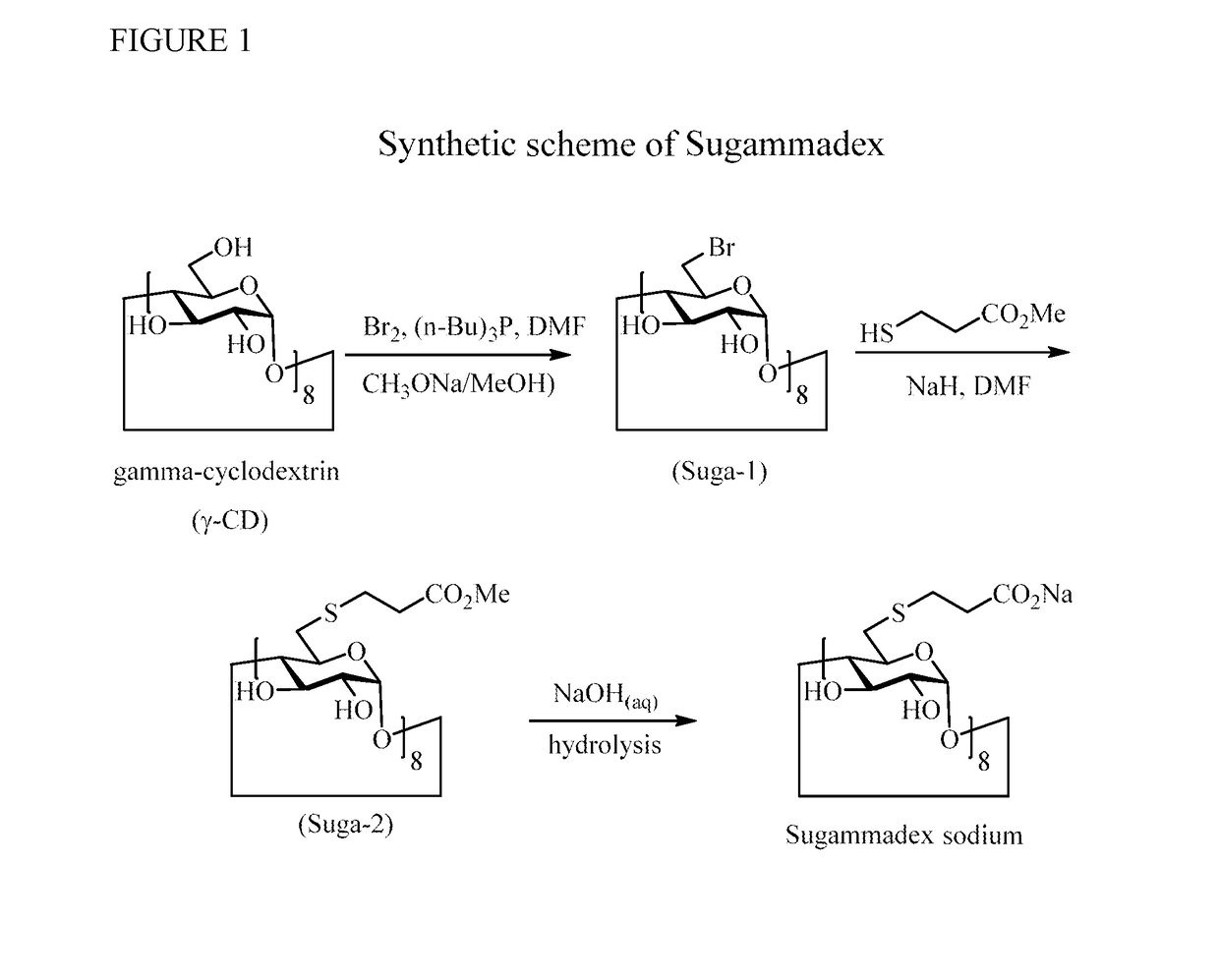
Method for preparation of sugammadex sodium
a technology of sugammadex and sodium, which is applied in the direction of mercapto/sulfide group formation/introduction, reverse osmosis, organic chemistry, etc., can solve the problems of triphenylphosphine oxide removal from the product, inconvenient large-scale operations, and high cost of purification techniques in the prior arts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of 6-per-deoxy-6-per-bromo-γ-cyclodextrin (Suga-1)
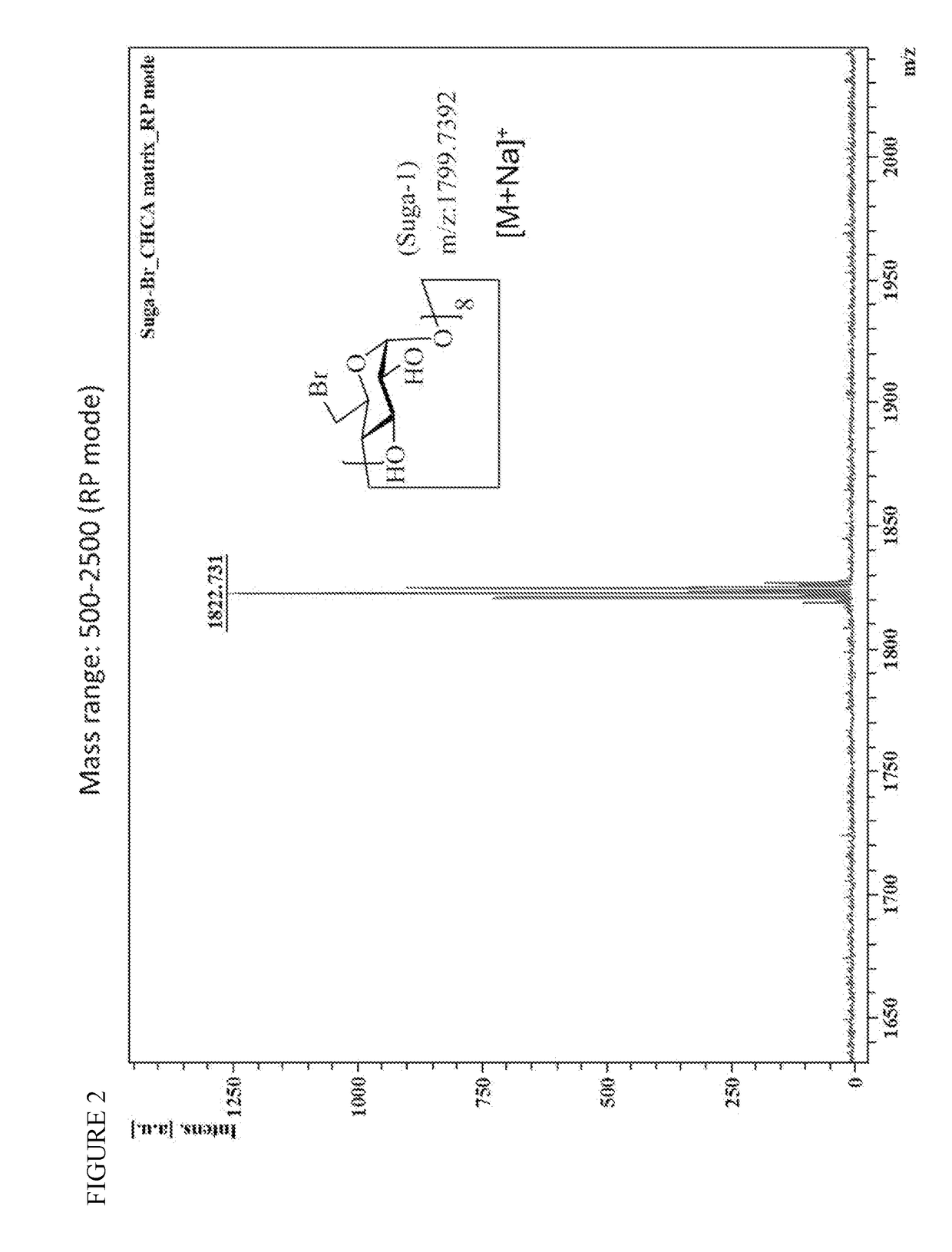
[0035]To a reaction flask, DMF (1.7 kg), Tributylphosphine (Bu3P) (540 g, 2.67 mol) were added and stirred about 5 min. Bromine (420 g, 2.63 mol) was added to the reaction flask, kept the temperature at 20° C. to 30° C. and stirred for 20 min, Gamma-cyclodextrin (180 g, 0.139 mol) was suspended in DMF (850 g), and added to the reaction flask. After the addition, reaction mixture was heated to about 70° C. and stirred for 4-6 hrs. 25% Sodium methoxide solution (580 g) and MeOH (480 g) was added to quench the reaction, concentrated to remove methanol, and then water (4.5 kg) was added to form the precipitate, The crude Suga-1 was washed with water (200 g).
[0036]To a reaction flask, isopropanol (IPA) (2.83 kg), crude Suga-1 were added. The reaction mixture was heated to 35° C. to 50° C., stirred for 1 hr to remove tributylphosphine oxide (TBPO), then recrystallization from DMF (380 g) / IPA (2.83 kg) solvent. The title compound (170 g)...
example 2
on of 6-per-(2-carboxyethyl)thio-γ-cyclodextrin, methyl ester (Suga-2)
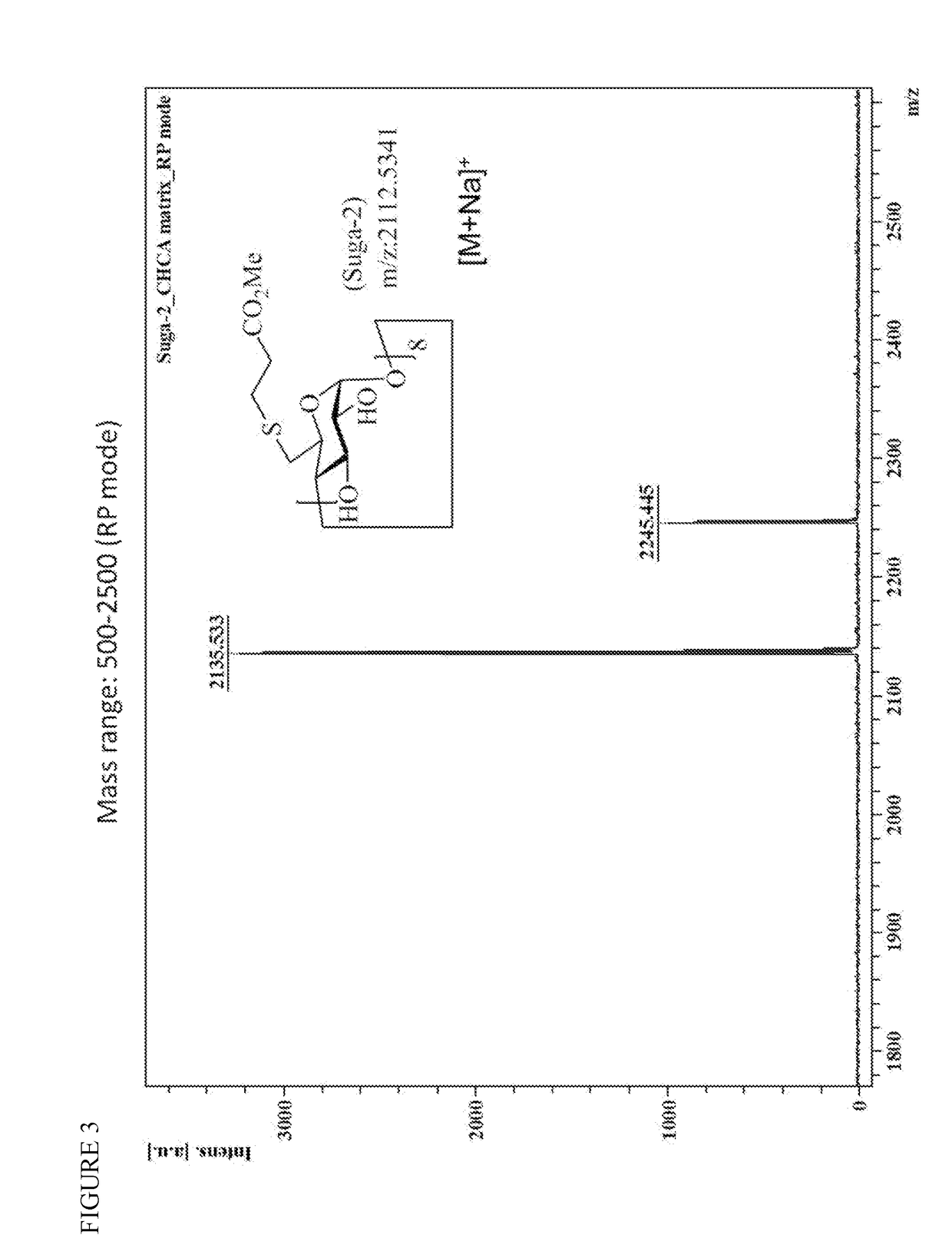
[0037]To a reaction flask, DMF (400 g), 60% NaH (50 g) were added under N2. After the addition, the reaction mixture was de-gased and purged with N2 iterally three times. Methyl 3-mercaptopropionate (210 g, 1.75 mol), DMF (400 g) were added to the reaction flask which were cooled to −10° C. to 0° C. during the period of addition. After the addition, the temperature of the reaction flask was risen to −5° C. to 0° C. Suga-1 (170 g, 0.97 mol) in DMF (810 g) solution was added to the reaction mixture and stirred for 1-1.5 hrs. The reaction was completed by check with HPLC. Water (2.88 kg), ammonium chloride (320 g) aqueous solution were added to quench the reaction. The precipitate was formed gradually. The mixture was filtered, and the crude Suga-2 was obtained after washed with water (400 g). The pure title compound (200 g) white powder was obtained by recrystallization from Acetonitrile (1.57 kg) / water (1 kg) solut...
example 3
on of 6-per-(2-carboxyethyl)thio-γ-cyclodextrin, Sodium Salt (Sugammadex Sodium)
[0038]To a reaction flask, IN NaOH (30 g), water (700 g) aqueous solution, Suga-2 were added under N2. The reaction mixture was stirred for 16 to 17 hrs. The pH of clear reaction solution was adjusted to 9 to 10 with 32% hydrochloride (10 g), water (140 g) aqueous solution, then methanol (1.5 kg) was added to form white powder of crude Sugammadex sodium. Pure wet Sugammadex sodium was obtained by recrystallization from methanol (330 g) / water (140 g) solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com