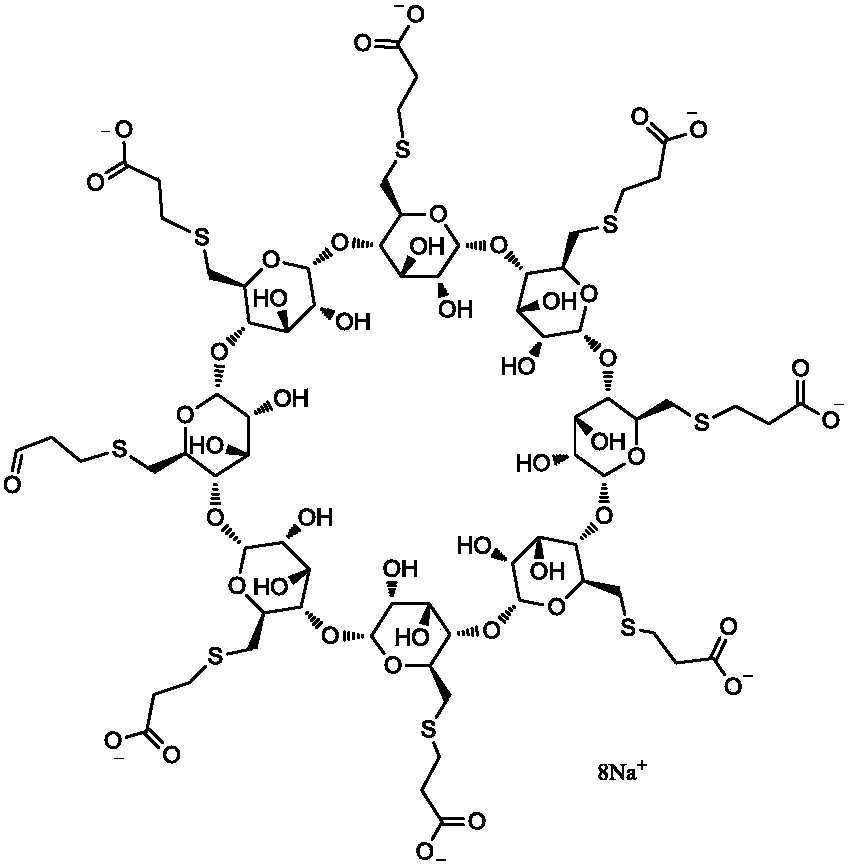
Purifying method of sugammadex sodium
A technology of sugammadex sodium and a purification method, applied in the field of high-purity sugammadex sodium, can solve the problems of inability to provide high-purity sugammadex sodium, complex structure of sugammadex sodium and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of crude sugammadex sodium
[0036] 3.50kg of γ-cyclodextrin was placed in a vacuum drying oven I, and the temperature was 95°C with a vacuum degree of ≦-0.08MPa and dried for 10 to 12 hours, and then packed into a double-layer plastic bag for use.
[0037] Add 9.12kg of triphenylphosphorus and 35kg of DMF into a 100L reaction kettle, protect with nitrogen, stir to dissolve, cool down to -15°C, add dropwise the DMF solution of iodine (9.14kg is dissolved in 11kg DMF), and continue to stir for 0.5-1h after dropping , and then add dry 3.00kg γ-cyclodextrin, the process control temperature does not exceed 0 ℃, and the temperature rises and stirs the reaction. The system was cooled, and the methanol solution of sodium methoxide (2.18kg sodium methoxide dissolved in 8kg methanol) was added dropwise, stirring was completed for 0.5 to 1 hour, and the material solution was transferred to a 50L plastic bucket.
[0038] Into the 500L reactor II, pump 300kg...
Embodiment 2
[0041] Packing the preparative column: 1.2kg silica gel C18 packing (20μm particle size, ) After wetting with 2.4L isopropanol, put it into the column tube of the preparation column, adopt dynamic axial compression, make the packing pressure reach 80~100Bar, and use 5~10 times CV (column volume) of ethanol as the mobile phase to flush the preparation column for backup.
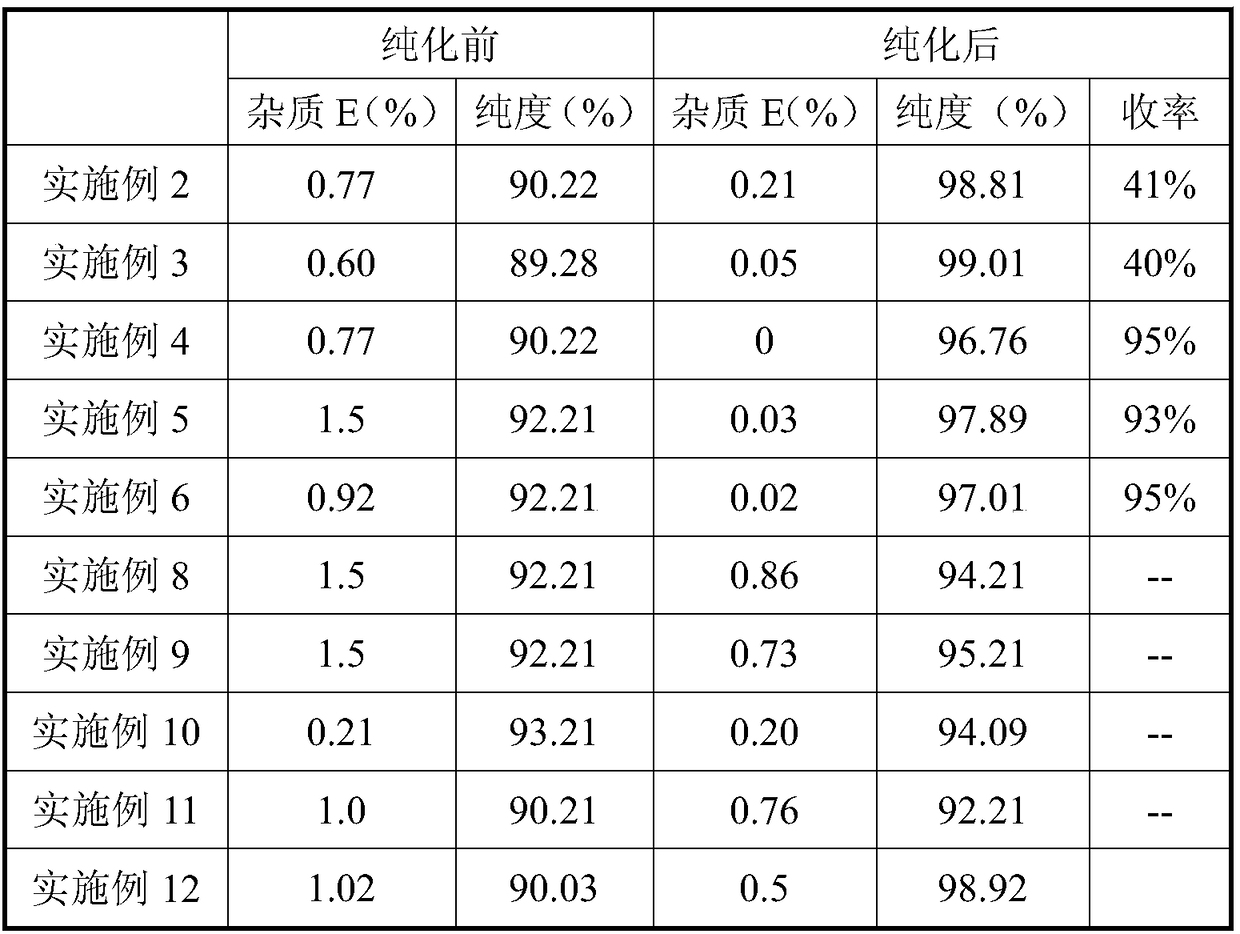
[0042] Dissolve 10 g of crude sodium gluconate in 100 ml of purified water in Example 1, filter with a 0.45 μm membrane filter, continue adding water to the system to 500 ml, and adjust the pH to be acidic.
[0043] The solution was loaded onto a preparative column, and 0.15% formic acid aqueous solution and acetonitrile were pumped to the chromatography column by pipelines A and B, respectively, for elution, keeping the acetonitrile content at 21%. The eluted main peak was divided into different fractions, and the qualified fractions were combined and concentrated to obtain 3.7 g of sodium sugammadex, the y...
Embodiment 3
[0045] Pack the preparative column according to Example 2 for use
[0046] Dissolve 10 g of crude sugammadex gluconate (purity 89.28%, impurity E content 0.60%) in 100 ml of purified water, filter with 0.45 μm filter membrane, continue adding water to the system to 500 ml, and adjust the pH to be acidic.
[0047] The solution was loaded onto a preparative column, and 0.15% formic acid aqueous solution and acetonitrile were pumped to the chromatography column by pipelines A and B, respectively, for elution, keeping the acetonitrile content at 21%. The eluted main peak was divided into different fractions, and the qualified fractions were combined and concentrated to obtain 3.6 g of sodium sugammadex, the yield was 40%, the purity was 99.01%, and the impurity E content was 0.05%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com