Preparation technology of sugammadex sodium
A technology of sugammadex sodium and sodium hydride, applied in the field of preparation of sugammadex sodium, can solve the problems of small difference in polarity, small difference in molecular weight, and high content of impurities related to sugammadex sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
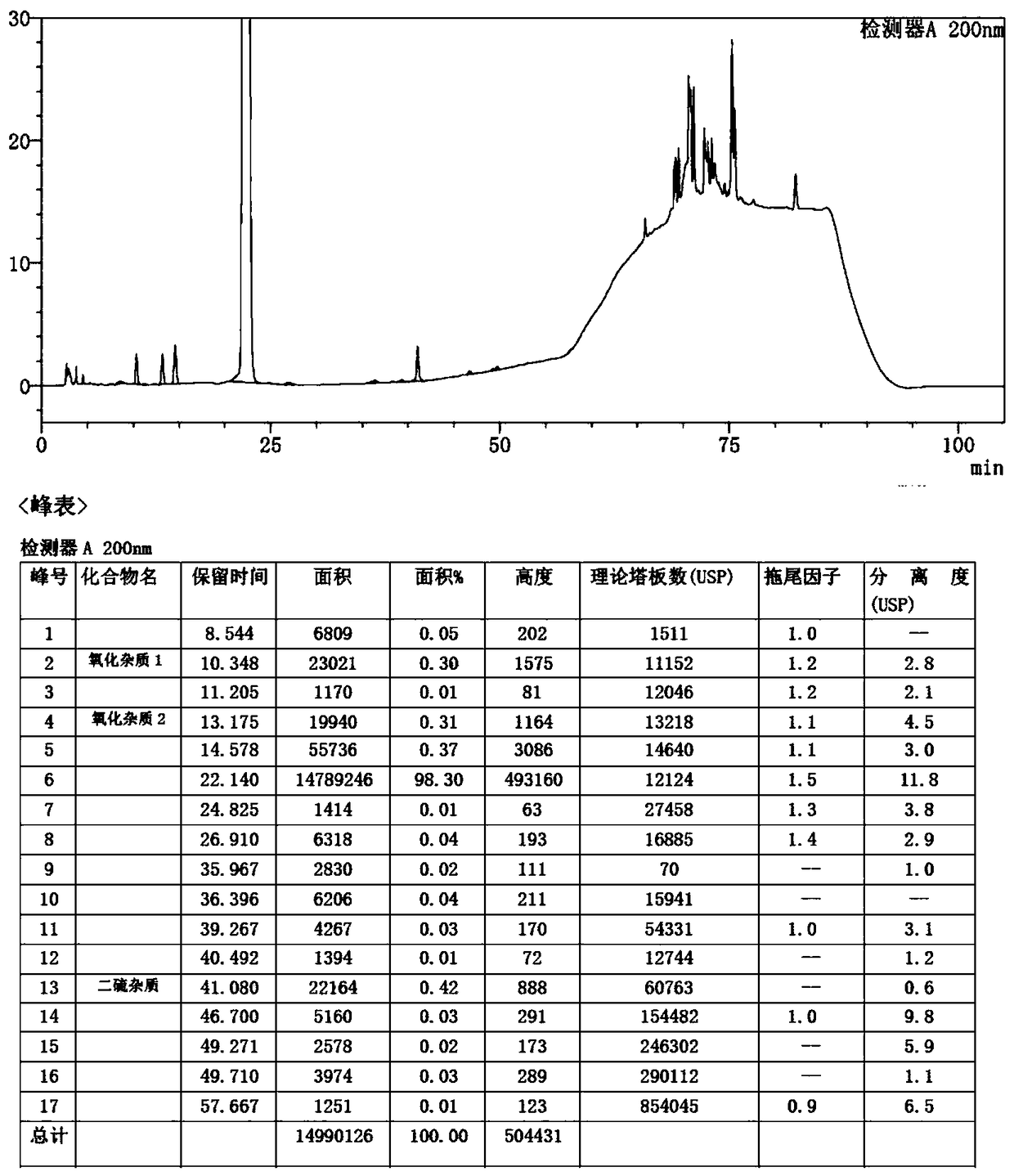
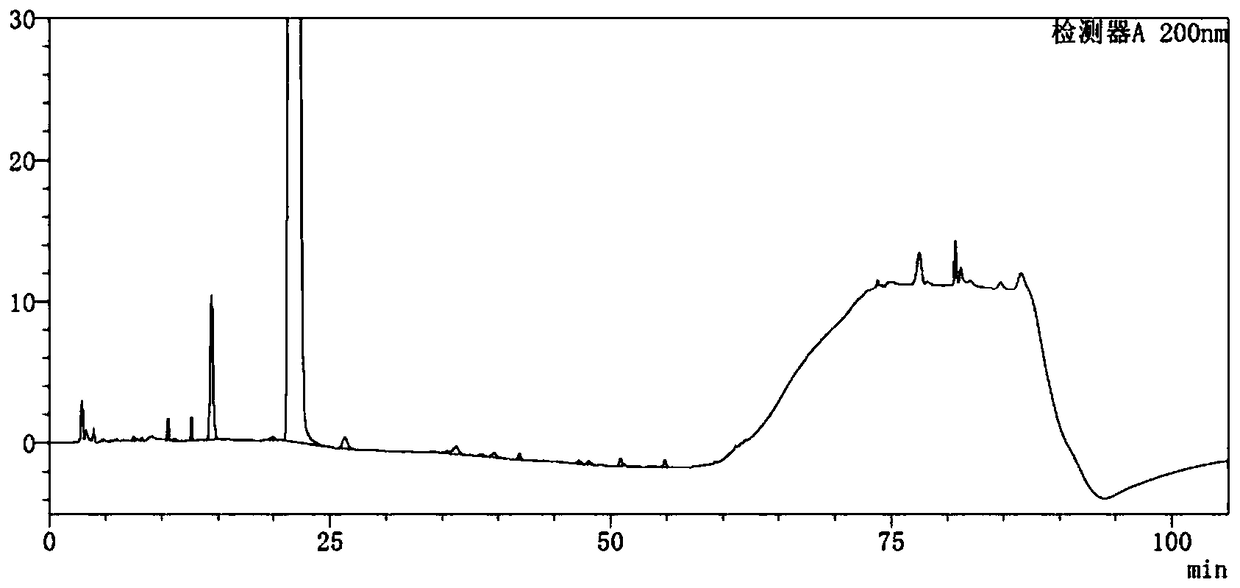
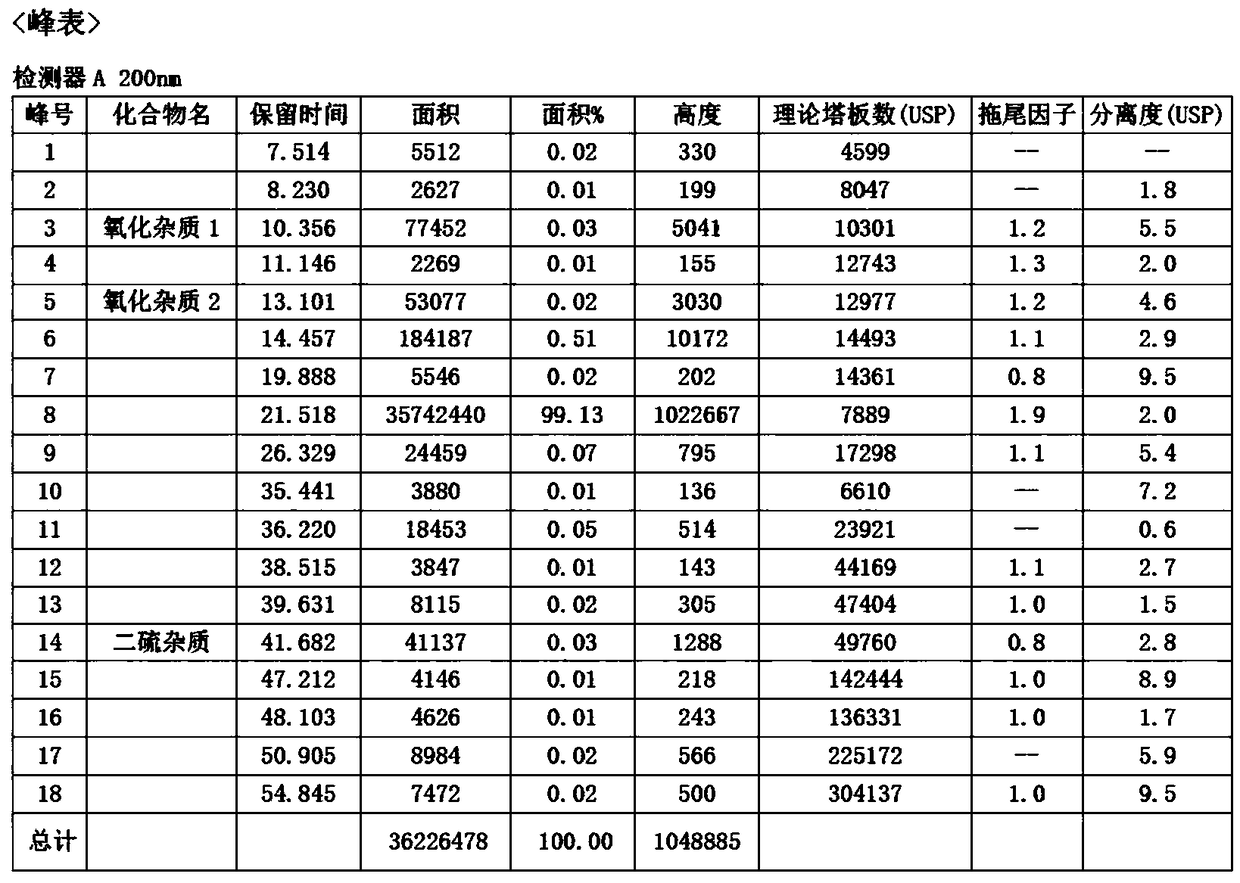
Image
Examples
Embodiment 1
[0028] Preparation of γ-ICD
[0029] In a 2000mL three-neck flask, under nitrogen protection, add 160ml of DMF and triphenylphosphine (30.1g, 15eq), and add iodine (30.5g, 15.6eq), exothermic violently, control the temperature at 25±5°C, and stir for 10min. Add dry good γ-cyclodextrin (10 g, 7.7 mmol), after the addition is complete, raise the temperature to 70° C. and stir for reaction for 24 hours. Cool the reaction solution to 10°C, add sodium methoxide solution (3.1g sodium added to 50ml methanol), stir for 30min, then add methanol 800ml, add 500ml purified water, and filter the solid. Crude γ-ICD 18.5 g was washed with water (3 x 100 ml) and then with acetone (3 x 100 ml).
[0030] In a 500ml three-necked flask, under nitrogen protection, add 18.5g of γ-ICD, add 185g of DMF, raise the temperature to 70°C, add 125g of purified water dropwise, the dropwise addition is complete, a large amount of solids are washed out, naturally and slowly cool down, and stir at 25°C for 4 ...
Embodiment 3
[0038] Add 1900g of DMF under nitrogen protection, add 60% sodium hydrogen (36.7g, 0.92mol), add BHT (0.88g, 0.004mol), cool to -5°C, add dropwise 3-mercaptopropionic acid (46.3g, 0.44mol) DMF solution, control the temperature at -5±5°C, complete the dropwise addition, raise the temperature to room temperature and stir for 2 hours, then add the 6-perdeoxy-6-periodo-γ-cyclodextrin obtained in Example 1 (95.0g, 0.04 mol) in DMF solution, heated up to 45°C, and reacted for 12h. After the reaction was completed, cooled to 5°C, added dropwise 558g of purified water, and filtered to obtain a crude product.
[0039] Dissolve the crude product obtained in the previous step in 400g of water, add 150g of diatomaceous earth and 1.0g of activated carbon, stir at room temperature for 30min and filter, transfer the filtrate to a 2000ml three-neck flask, replace with nitrogen three times, add 1200g of DMF solution dropwise, and stir at room temperature for 3 hours More sodium dextrose wet pr...
Embodiment 4
[0042] Under the protection of nitrogen, add 1900g of DMF, add 60% sodium hydrogen (36.7g, 0.92mol), add BHT (0.44g, 0.002mol), cool to -5 ° C, dropwise add 3-mercaptopropionic acid (46.3g, 0.44mol) DMF solution, control the temperature at -5±5°C, complete the dropwise addition, raise the temperature to room temperature and stir for 2 hours, then add the 6-perdeoxy-6-periodo-γ-cyclodextrin obtained in Example 1 (95.0g, 0.04 mol) in DMF solution, heated up to 45°C, and reacted for 12h. After the reaction was completed, cooled to 5°C, added dropwise 558g of purified water, and filtered to obtain a crude product.
[0043] Dissolve the crude product obtained in the previous step in 400g of water, add 150g of diatomaceous earth and 1.0g of activated carbon, stir at room temperature for 30min and filter, transfer the filtrate to a 2000ml three-neck flask, replace with nitrogen three times, add 1200g of DMF solution dropwise, and stir at room temperature for 3 hours More sodium dextr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com