Medical usage of 2beta-hydroxyilicicacid in inhibiting hepatitis B
A technology of hydroxypectinic acid and hepatitis B virus, applied in the field of eucalyptane-type sesquiterpene derivative 2β-hydroxypectinic acid, can solve the problems of reducing hepatitis B surface antigen, and there is no anti-hepatitis B virus drug for hepatitis B virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
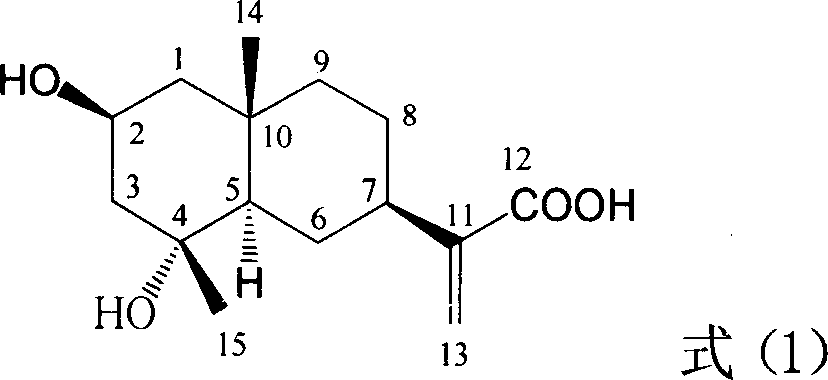
[0020] The preparation method of the eucalyptane type sesquiterpene acid shown in this formula (1) can be referred to the article that the researcher such as inventor has published [Zheng Qunxiong, Zhang Qijun, Zhao Yu etc., Zhejiang University Journal (Medical Edition), 2002,31 (6): 406: Zhao Yu (Zhao Y) et al. Journal of Natural Products, 1997, 60(6): 545]. The compound of formula (1) was prepared according to the method described in the literature, and the spectral data of the purified compound of formula (1) was consistent with the value reported in the literature.
[0021] Spectral data of the compound of formula (1): colorless needles, melting point 198-199°C (methanol); mass spectrum EIMS m / z[M] + 268(7), 250, 217, 204, 167; 13 C-NMR (100MHz, deuterated acetone) δ170.04, 148.39, 121.52, 70.86, 67.82, 55.56, 50.23, 47.82, 45.92, 41.39, 34.75, 27.63, 27.36, 25.63, 20.84.
[0022] The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth was determin...
Embodiment 1
[0024] Example 1 Inhibitory effect of formula (1) compound on the hepatitis B surface antigen (HBsAg) secreted by HepG2.2.15 cells
[0025] 1) Cell culture:
[0026] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin, 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0027] 2) The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth was measured by MTT method:
[0028] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 After cultivating in an incubator with 100% relative humidity for 24 hours, add the compound of formula (1) diluted with medium, the concentration is respectively 1000 μg / ml, 200 μg / ml, 40 μg / ml and 8 μg / ml, 200 μl in each well, each Set the concentration in three duplicate wells, cult...
Embodiment 2
[0036] Example 2 : Inhibitory effect of formula (1) compound on the replication of hepatitis B virus deoxyribonuclease (HBV-DNA) secreted by HepG2.2.15 cells.
[0037] 1) Cell culture:
[0038] Method is with embodiment 1.
[0039] 2) The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth was measured by MTT method:
[0040] Method is with embodiment 1.
[0041] 3) Determination of the inhibitory effect of the compound of formula (1) on the replication of hepatitis B virus deoxyribonucleic acid (HBV-DNA).
[0042] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 After cultivating in an incubator with 100% relative humidity for 24 hours, add the compound of formula (1) diluted with medium, the concentration is respectively 100 μg / ml, 20 μg / ml and 40 μg / ml, 200 μl per well, and three concentrations are set for each Dupli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com