Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
A technology of hydroxypearic acid and hepatitis B virus, applied in antiviral agents, drug combinations, digestive system, etc., can solve problems such as reducing hepatitis B surface antigen, and there are no anti-hepatitis B virus drugs for hepatitis B virus infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
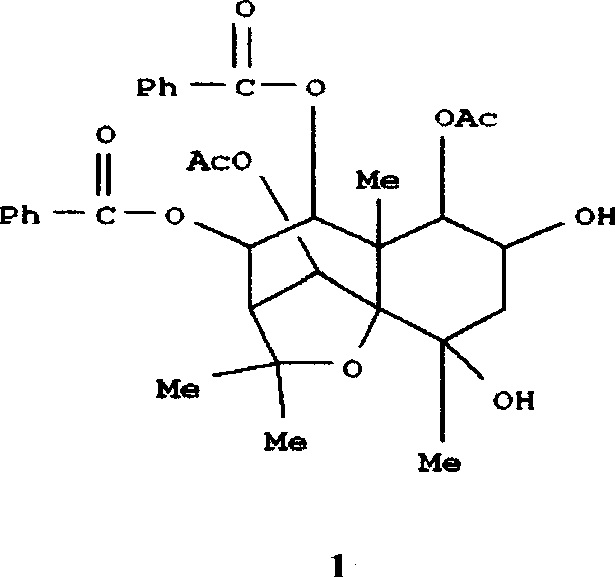
[0019] For the preparation method of the eucalyptane-type sesquiterpene acid represented by the formula (1), see the published articles by researchers such as the inventor (Ous, L. et al. Nat Prod Lett 1998, 12:231; Zheng Qunxiong, Zhao Yu et al., J Nat Prod, 2003, 66(8): 1078.) The compound of formula (1) was prepared according to the method described in the literature, and the spectral data of the compound of formula (1) obtained by purification were consistent with the reported values in the literature.
[0020] The spectral data of the compound of formula (1) we obtained are as follows: colorless needle crystal, melting point: 172-173°C (methanol); 1 H-NMR (deuterated pyridine, 400 MHz) δ 6.24 (1H, brs), 5.74 (1H, brs), 3.66 (1H, dd, J=12.5, 4.0 Hz), 2.91 (1H, ddd, J=13.0 , 12.5, 4.0, 4.0 Hz), 2.64 (1H, ddd, J=13.5, 13.0, 3.6 Hz), 2.36 (1H, ddd, J=13.5, 4.5, 2.5 Hz), 1.98 (1H, m), 1.96 ( 2H, m), 1.82 (1H, m, ), 1.72 (1H, dd, J=13.0, 3.6 Hz), 1.70-1.90 (2H, m), 1.64 (1H,...
Embodiment 1
[0022] Example 1 : Inhibitory effect of compound of formula (1) on hepatitis B surface antigen (HBsAg) secreted by HepG2.2.15 cells
[0023] 1) Cell culture:
[0024] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100U / ml penicillin, 100μg / ml streptomycin, and 100μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity. 2) The inhibitory effect of the compound of formula (1) on the growth of HepG2.2.15 cells was determined by MTT method:
[0025] Take HepG2.2.15 cells in logarithmic growth phase and dilute the cells to 1 × 10 with culture medium 5 / ml, seeded in 96-well cell culture plates, 100 μl per well, at 37°C, 5% CO 2 , after culturing in a 100% relative humidity incubator for 24 hours, the compound of formula (1) diluted with culture medium was added at concentrations of 1000 μg / ml, 200 μg / ml, 40 μg / ml and 8 μg / ml, 200 μl per well, each The concentration was set up in three duplicate wells, place...
Embodiment 2
[0033] Example 2 : Inhibitory effect of the compound of formula (1) on the replication of hepatitis B virus deoxyribonucleic acid (HBV-DNA) secreted by HepG2.2.15 cells
[0034] 1) Cell culture:
[0035] The method is the same as in Example 1.
[0036] 2) The inhibitory effect of the compound of formula (1) on the growth of HepG2.2.15 cells was determined by MTT method:
[0037] The method is the same as in Example 1.
[0038] 3) To determine the inhibitory effect of the compound of formula (1) on the replication of hepatitis B virus deoxyribonucleic acid (HBV-DNA).
[0039] Take HepG2.2.15 cells in logarithmic growth phase and dilute the cells to 1 × 10 with culture medium 5 / ml, seeded in 96-well cell culture plates, 100 μl per well, at 37°C, 5% CO 2 , after culturing for 24 hours in an incubator with 100% relative humidity, the compound of formula (1) diluted with the medium was added at the concentrations of 100 μg / ml, 20 μg / ml and 40 μg / ml, 200 μl per well, and each...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com