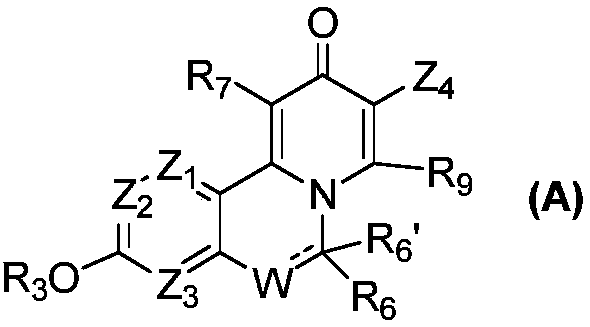
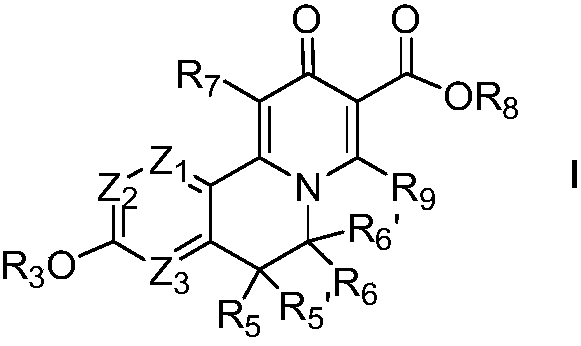
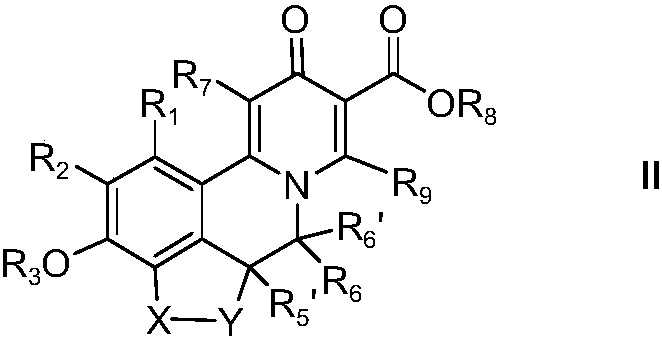
Novel isoquinoline compound and medical application thereof
A technology of compounds and medicinal salts, which is applied in the field of new isoquinoline compounds and their medical applications, can solve the problem of not being able to reduce hepatitis B surface antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: 6-isopropyl-10-methoxy-9-(3-methoxypropoxy)-N-(cyclopropylsulfonyl)-2-oxo-6,7-dihydro -2H-pyrido[2,1-a]isoquinoline-3-carboxamide (10)
[0098]
[0099] Step 1: Synthesis of 4-bromo-1-methoxy-2-(3-methoxypropoxy)benzene (2)
[0100]
[0101] 1-Bromo-3-methoxypropane (49.8g, 325.4mmol) was added dropwise to a solution of 5-bromo-2-methoxyphenol (22.0g, 108.4mmol) and anhydrous potassium carbonate (45g, 325.6mmol) in DMF (30mL) suspension, stirred overnight at room temperature, poured into water, extracted three times with ethyl acetate, combined organic phase, washed with water and saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid, 26g, 87.2% . ESI-MS: [M+H]+: 275.2.
[0102] Step 2: 1-(4-methoxy-3-(3-methoxypropoxy)phenyl)-3-methylbutan-2-one (3)
[0103]
[0104] In the reaction flask of THF (180mL), add compound 2 (20.6g, 75.1mmol), Pd2(dba)3(1.38g, 1.5mmol), xantphos (1.74g, 3.0mmol), sodium tert...
Embodiment 2
[0126] Example 2: 6-isopropyl-10-methoxy-9-(3-methoxypropoxy)-3-(1H-tetrazol-5-yl)-6,7-dihydro-2H -pyrido[2,1-a]isoquinolin-2-one (12)
[0127]
[0128] Reaction process 2:
[0129]
[0130] Step 1: 6-Isopropyl-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7-dihydro-2H-pyrido[2,1-a ] Synthesis of isoquinoline-3-carboxamide (11)
[0131]
[0132]Compound 9 (1g, 2.49mmol) was added to a reaction flask of thionyl chloride (10mL), dripped into 1d DMF, refluxed for 3 hours, concentrated, and the residual thionyl chloride was removed with toluene, then diluted with THF (10mL), Cool to 0°C, add ammonia water (5 mL) dropwise, raise to room temperature, stir overnight, evaporate THF under reduced pressure, filter the obtained solid, wash with ice water to obtain compound 11, 0.86 g, 87%. Proceed directly to the next reaction. ESI-MS: [M+H]+: 401.2.
[0133] Step 2: 6-Isopropyl-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7-dihydro-2H-pyrido[2,1-a ] Synthesis of isoquinoline-3-carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com