Hepatitis A virus monoclonal antibody and its application
A technology of monoclonal antibody and hepatitis A virus, applied in the direction of antibody, antiviral agent, antiviral immunoglobulin, etc., can solve the problem of rare transmission mode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The acquisition of embodiment 1 hybridoma cell line
[0024] Including the following steps:
[0025] 1. Preparation of immunogen
[0026] The immunogen is hepatitis A virus antigen --- TZ84 strain, provided by Beijing Kexing Biological Products Co., Ltd. The virus was inoculated into 2BS cells, cultured and harvested, purified by PEG precipitation and chloroform extraction, and then concentrated and inactivated by ultrafiltration to prepare purified virus liquid.
[0027] 2. Animal immunity
[0028] BALB / c mice were initially immunized and boosted with purified hepatitis A virus liquid, and the BALB / c mice used were female, aged 6-8 weeks, and weighed 18-22g. Immunization procedure: For the first immunization, the purified hepatitis A virus was mixed and emulsified with Freund's complete adjuvant, and immunized with BALB / c mice, 150 μg / mouse, and injected subcutaneously at multiple points; the second immunization was performed after an interval of 10 days, and the pu...
Embodiment 2
[0035] Example 2 Screening and Preparation of Monoclonal Antibodies
[0036] 1. Preparation of monoclonal antibody in ascites
[0037] Ascites was prepared by intraperitoneal injection of mice for hybridoma cell expansion. The ascites preparation process is as follows: culture hybridoma cells, inject paraffin-sensitized BALB / c mice in the retroperitoneal cavity, collect ascites at about 7 to 14 days, centrifuge at 1200 rpm to remove sediment, and collect the upper layer of monoclonal antibody ascites.
[0038] 2. Preliminary screening and preparation of monoclonal antibodies
[0039] Dilute 1:100 with hepatitis A virus purification solution and coat the microplate respectively, 100μl / well, coat overnight; wash the plate 3 times with 0.01M PBST-20 (containing 0.5v / v‰Tween-20) washing solution , add 10% calf serum, 0.01M PBST-20 solution (containing 0.5v / v‰Tween-20), 200μl / well, block at 37°C, discard the solution in the plate. Add serially diluted monoclonal antibody ascites...
Embodiment 3
[0042] Example 3 Monoclonal antibody subtype identification
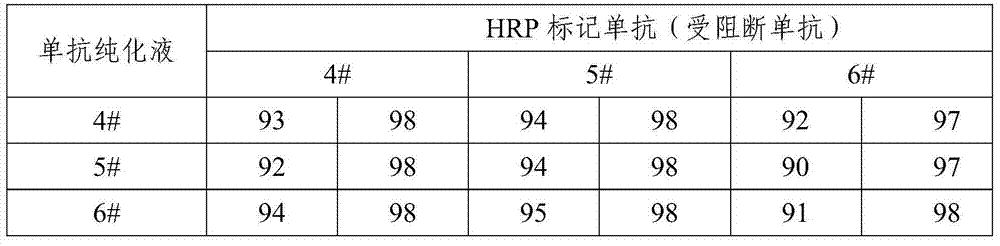
[0043] According to the instructions of the Antibody Subtype Identification Kit (purchased from Southern Biotech), the subtype of the monoclonal antibodies screened in Example 2 was identified. The experimental results are shown in Table 1.
[0044] Table 1 The subtype identification results of 6 monoclonal antibodies against hepatitis A
[0045]
[0046] It can be seen from Table 1 that 1# monoclonal antibody is of IgM type, and the specificity of this subtype antibody is poor; 2# monoclonal antibody is of IgG3 type, and this subtype antibody is not easy to purify under limited experimental conditions; therefore 1#, 2# monoclonal antibody Not suitable as raw material for ELISA enzyme-labeled antibody. Among the remaining 4 monoclonal antibodies, 3# and 5# monoclonal antibodies are of IgG2a type, and 4# and 6# monoclonal antibodies are of IgG2b type, which can be used as candidates for ELISA enzyme-labeled anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com