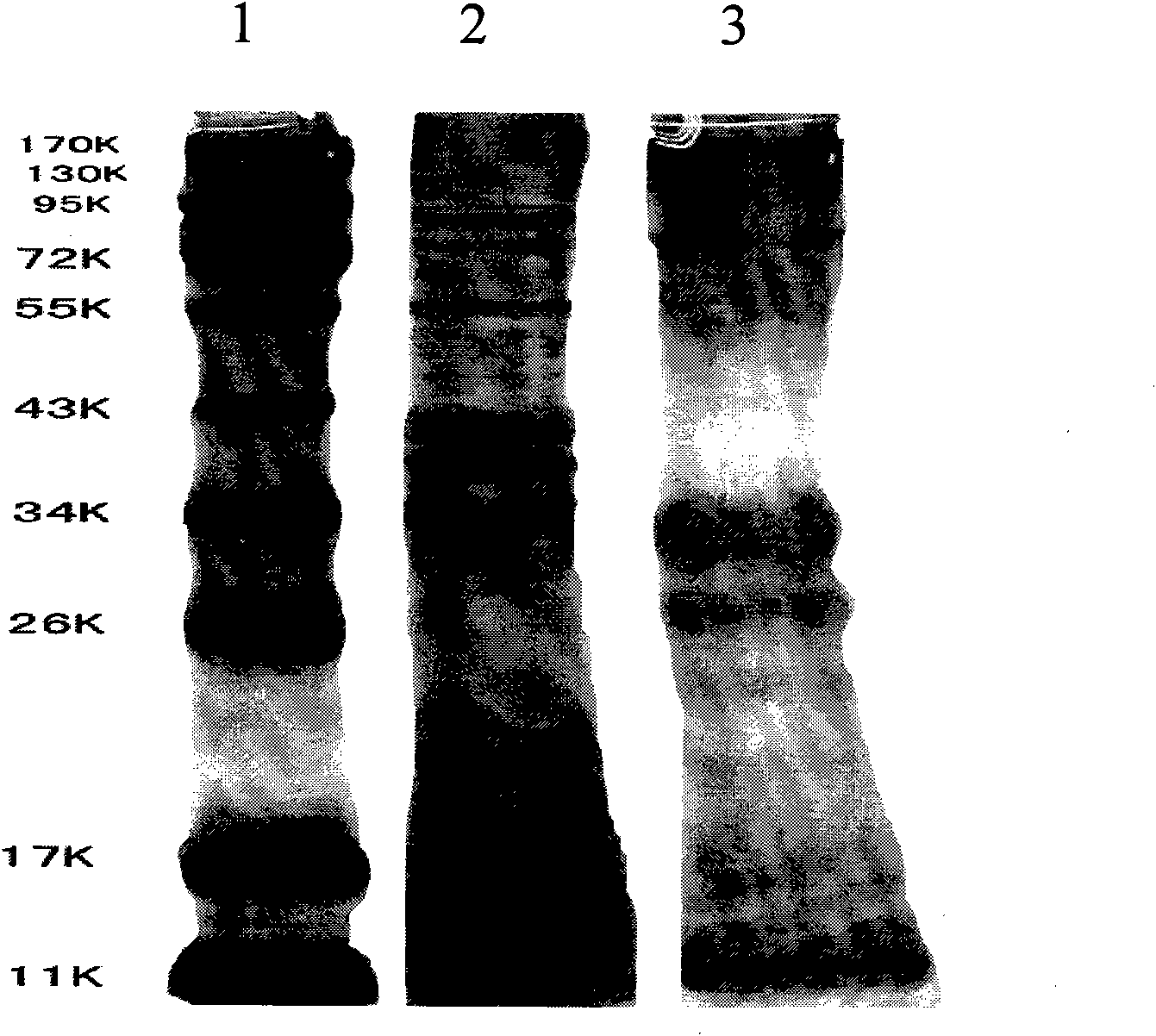
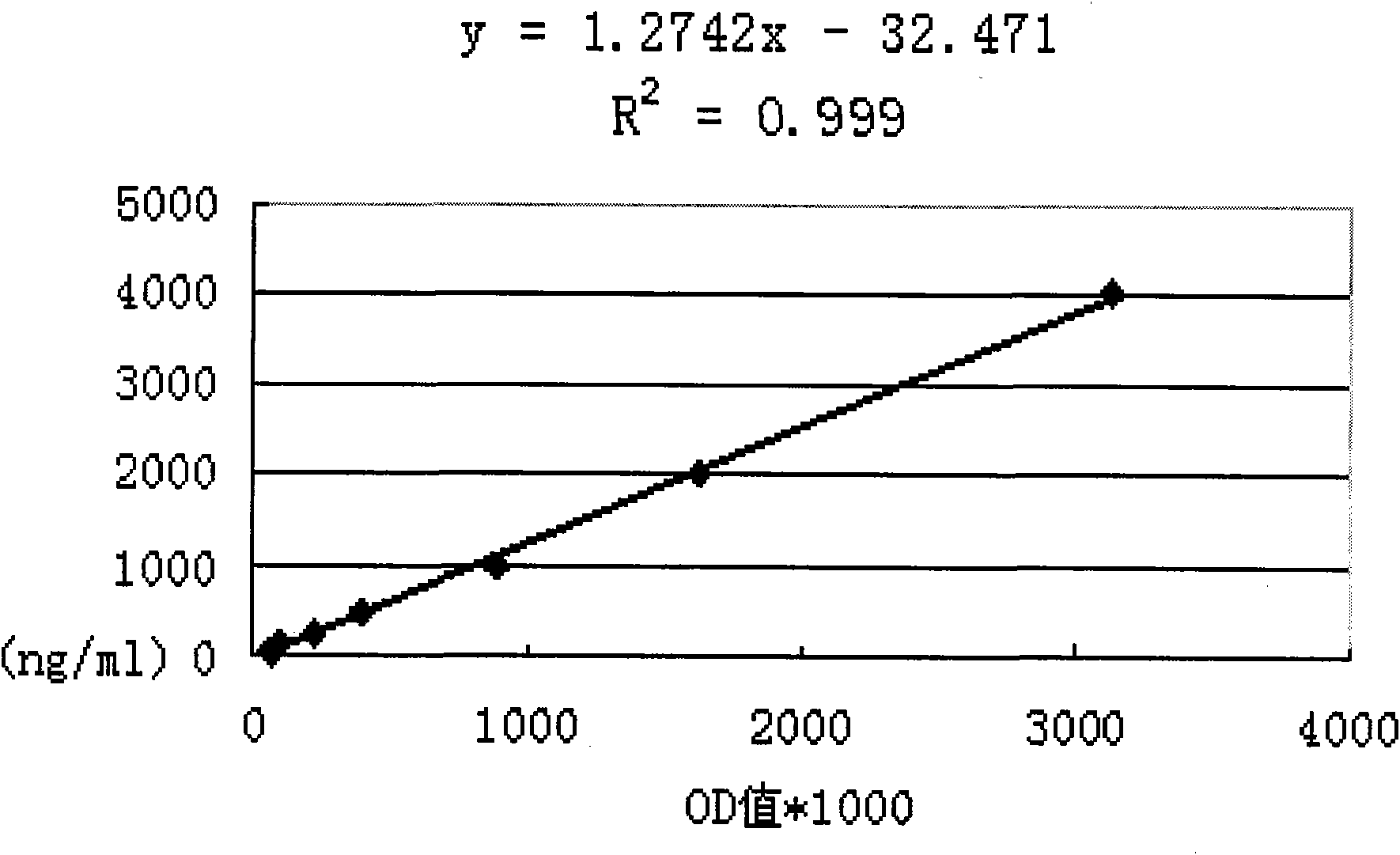
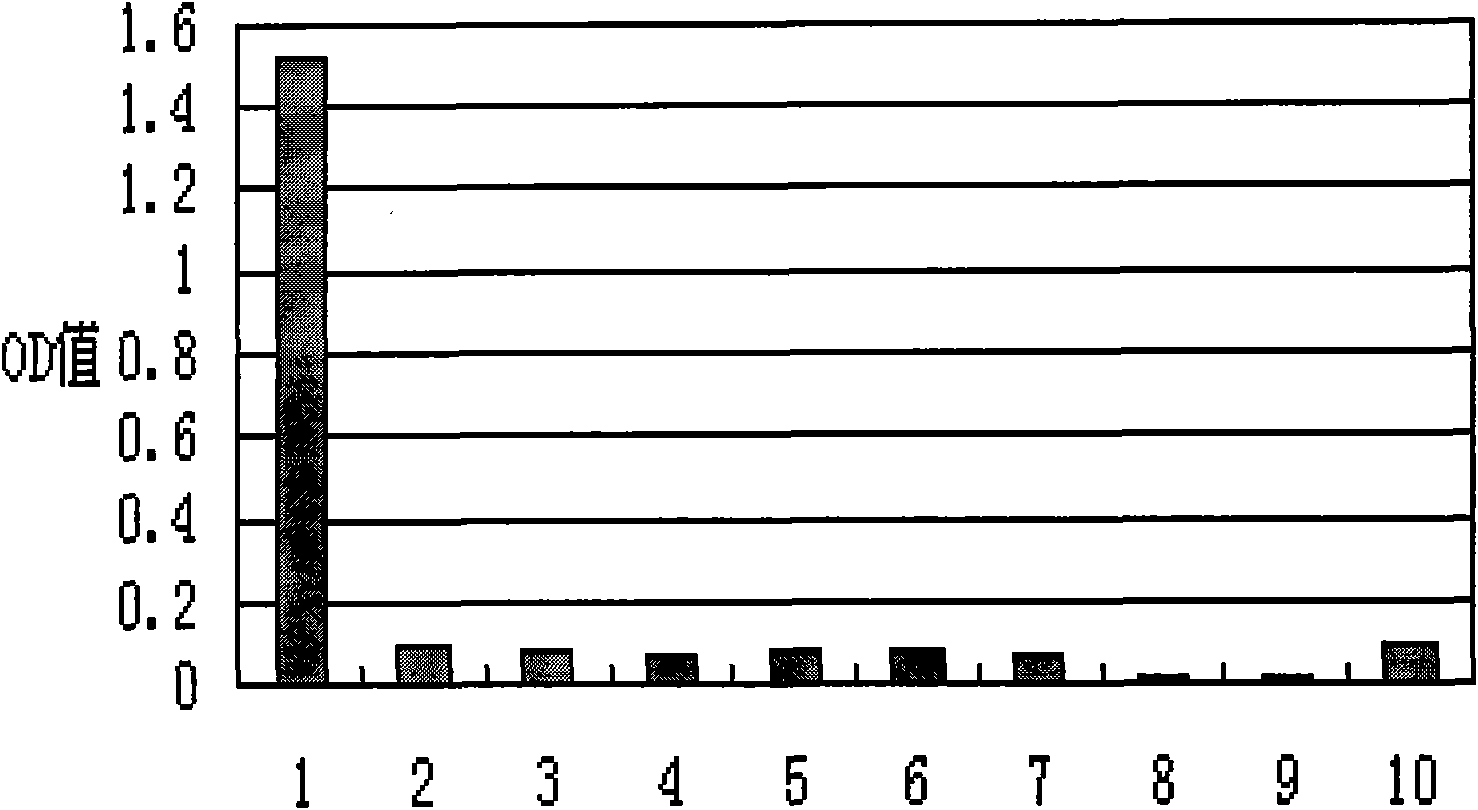
Vero cell HCP test kit and application thereof
A detection kit and cell technology, applied in the biological field, can solve problems such as insufficient detection methods, and achieve high sensitivity, strong specificity, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The anti-Vero cell lysate protein antibody can be prepared by using Vero cell lysate protein as an antigen, and various corresponding antibodies can be obtained by the following methods, but not limited to the following methods.
[0045] Such as rabbit anti-Vero cell lysis protein IgG and guinea pig anti-Vero cell lysis protein IgG, its preparation method is as follows:
[0046] 1) Immunize three rabbits. For initial immunization, add 0.5 mg of Vero cell lysate protein prepared in Example 1 to each rabbit, add an equal volume of Freund's complete adjuvant, emulsify fully, and inject subcutaneously on the back at 6-8 points. The second immunization after 4 weeks: use 0.5 mg of purified Vero cell lysate protein to add an equal volume of Freund's incomplete adjuvant, and after emulsification is sufficient, inject subcutaneously and intramuscularly at multiple points. The third immunization was carried out in the same way 10 days after the second immunization. Finally, the...
Embodiment 1
[0076] Embodiment 1: the preparation of Vero cell lysate protein
[0077] Preparation:
[0078] 1. Cell culture harvest: Take a Vero cell line cell tube from the working cell bank, inoculate the cells into a cell culture flask, add 199 culture medium containing 10% calf serum, and culture at 37±1°C. After the revived cells grew into a dense monolayer, the Vero cells were digested with trypsin for passage and expansion, cultured at 35±1°C for 25 days, and harvested. Digest with trypsin, centrifuge at 3500rpm, 4°C for 30 minutes, and collect the cell pellet. Used as a sample source for lysed protein preparation.
[0079] 2. Cell disruption and lysis: Harvested Vero cells were disrupted and lysed by ultrasonic method, with an output power of 1400W, the cells were broken until there was no complete cell structure.
[0080] 3. Chloroform extraction of protein: add an equal volume of chloroform to the crushed cell fluid, shake on a reciprocating shaker at a rate of 300rpm for 20m...
Embodiment 2V
[0088] Embodiment 2 Vero cell lysate protein antibody preparation
[0089] 1. Immunization of rabbits:
[0090] Three rabbits were immunized, each rabbit was first immunized with 0.5 mg Vero cell lysate protein prepared in Example 1, added with an equal volume of Freund's complete adjuvant, after emulsification was sufficient, the back was subcutaneously injected at 6-8 points. The second immunization after 4 weeks: add an equal volume of Freund's incomplete adjuvant with 0.5 mg of Vero cell lysate protein prepared in Example 1, and after emulsification is sufficient, inject subcutaneously and intramuscularly at multiple points. The third immunization was carried out in the same way 10 days after the second immunization. Finally, the fourth immunization was carried out 2 weeks after the third immunization, blood was collected one week later, and the titer was determined.
[0091] Table 2 Serum titers of immune rabbits
[0092]
[0093] 2. Immunization of guinea pigs:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com