Antibody and encoding gene of mycobacterium tuberculosis Acr protein and application thereof
A gene and protein technology, applied to the antibody of Mycobacterium Acr protein and its encoding gene and application field, can solve problems such as latent infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the acquisition of the antibody of Acr protein
[0033] 1. Prokaryotic expression of Mycobacterium tuberculosis Acr protein
[0034] ①Take the genome of MTB H37RV strain (Expression and purification of ACR protein of Mycobacterium tuberculosis in E.coli.Chinese Journal of Preventive Veterinary Medicine, 2009.Vol.31 No.7pp.528-531) as a template, and use the synthetic primers (sequence as follows :primer -f:
[0035] 5'-GGATCCAAAATGGCCACCACCCCTTCCCGTT-3', primer-r:
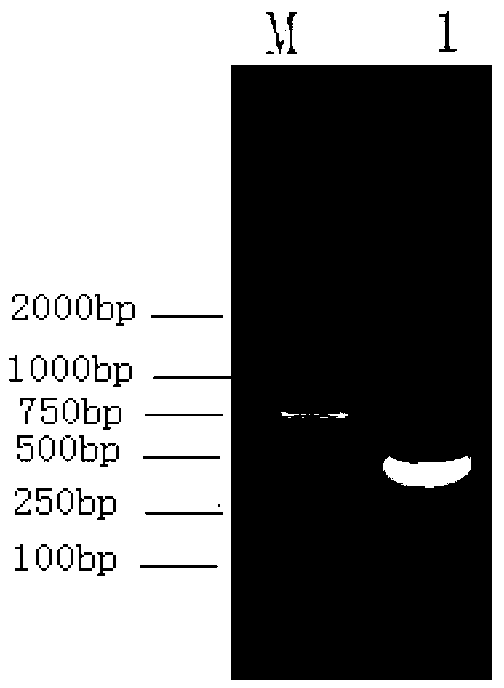
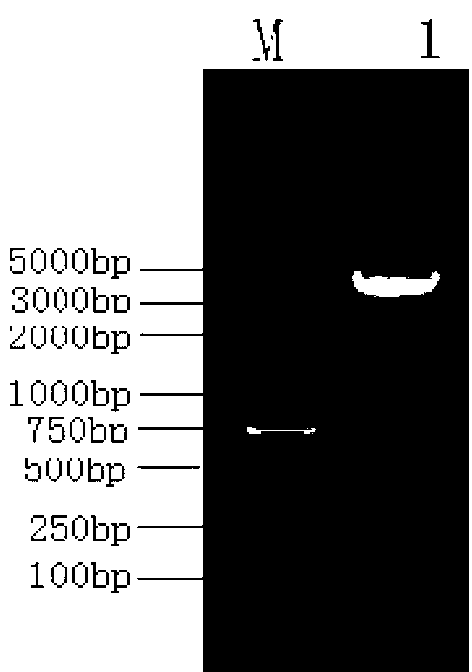
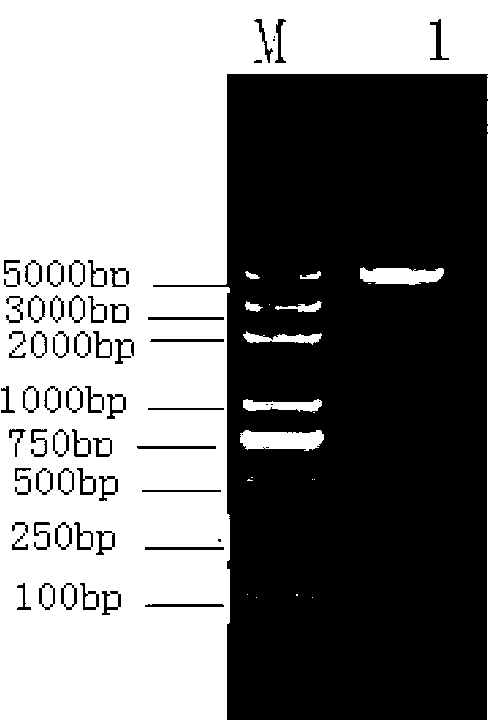
[0036] 5'-GGAAGCTTCAGTTGGTGGACCGGATCTGAATGTG-3) for PCR amplification, successfully amplified a PCR product of 435bp with the expected length of Mycobacterium tuberculosis Acr ( figure 1 , M: 2000bp DNA Marker, 1: PCR product).
[0037] The PCR product was connected to the peasy-blunt simple vector (Beijing Quanshijin Company, catalog number CB111-01) to obtain a recombinant vector, and the recombinant vector was digested with BamHI and Hind III to obtain a 435bp fragment as a positive recom...
Embodiment 2
[0062] Embodiment 2, the functional verification of the antibody of Acr protein
[0063] 1. Human antibody neutralization activity experiment
[0064] (1) Coating: the purified Acr protein obtained in Example 1, ovalbumin (OA) (purchased from sigma company product number: A5503-1G), SC protein (amino acid sequence is sequence 30), HIV-1 core protein P24 protein (The amino acid sequence is sequence 31) Antigens were diluted with coating solution to 2.5 μg / ml, 100 μl was added to each well of a polystyrene enzyme-linked detection plate, and coated overnight at 4°C.
[0065] (2) Blocking: add 200 μl per well or top up with blocking solution (5% skimmed milk powder), after 2 hours at 37°C, wash 3 times with PBST (0.05% Tween20), and pat dry.
[0066] (3) Add the sample to be tested: take the soluble single-chain antibody scFv1-scFv14 obtained in the third step of Example 1, add 100 μl / well to the coated microtiter plate, at the same time, incubate at 37°C for 30 minutes, and wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com