Enterovirus 71 type viral strain, its application, vaccine and preparation method
A technology of enteroviruses and virus strains, applied in antiviral immunoglobulins, biochemical equipment and methods, antiviral agents, etc., can solve the differences in cross-protection levels of virus strains, unclear pathogenic mechanisms, and difficulties in the development of EV71 vaccines To achieve effective immune activity and protection against viruses, good cross-immunogenicity, and easy control of the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Another aspect of the present invention provides a vaccine preparation method, which includes: culturing EV71 virus strain 1 or EV71 virus strain 2, inactivating and purifying, thereby preparing the vaccine. Preferably, said purification is performed before said inactivation and / or after said inactivation.
[0054] Preferably, the preparation method of the present invention comprises:
[0055] 1) Provide Vero cells or human diploid cells;
[0056] 2) culturing EV71 virus strain 1 or EV71 virus strain 2 in the Vero cells or human diploid cells obtained in step 1), thereby obtaining a virus suspension;
[0057] 3) purifying and inactivating the virus suspension obtained in step 2), so as to obtain the vaccine stock solution; and
[0058] 4) Diluting the vaccine stock solution obtained in step 3) to obtain the vaccine.
[0059] Herein, Vero cells refer to passaged African green monkey kidney cells, which can be obtained from ATCC; human diploid cells are derived from no...
Embodiment 1
[0081] Embodiment 1: the separation and identification method of EV71 virus strain 1
[0082] (1) Isolation method of EV71 virus strain 1
[0083] Candidate strains must have complete records, history, and sources. Determine the biological characteristics of candidate virus strains. The inactivated vaccines prepared by candidate virus strains should have high virus yield, strong ability to induce immune protection, broad cross-protection spectrum, and stable biological characteristics, and should be based on epidemiology and molecular epidemiology Selected virus strains with broad current and future epidemic potential. Animals are immunized with candidate viruses, serum is separated, serum neutralizing antibody titers are determined, and viruses with a broad protective spectrum and strong ability to induce immune protection are screened as inactivated vaccine virus strains.
[0084] Throat swab specimens were collected from a patient with HFMD (Huang XX, a 4-year-old HFMD ch...
Embodiment 2
[0089] Embodiment 2: Purification and passage stability analysis of EV71 virus strain 1
[0090] (1) Plaque purification
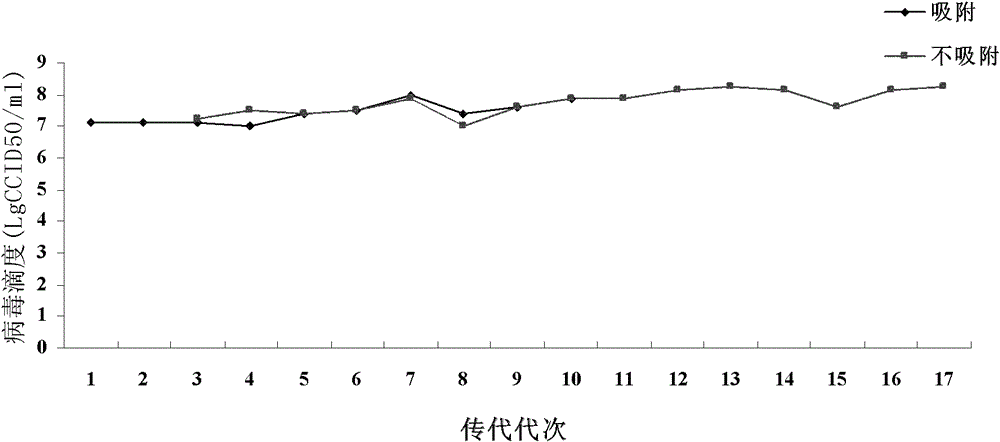
[0091] The EV71 virus suspension obtained in Example 1 was serially diluted, and after the cells were observed to grow into a single layer under a microscope, the original culture medium in the six-hole cell culture plate was discarded, and 0.1-0.5ml / hole of the virus suspension was added, 36±1 Adsorb at ℃ for 1-2 hours. After the adsorption is over, pour off the liquid in the plate, wash once with sterile PBS, add a cover, and culture in a carbon dioxide incubator at 36±1℃. 3-7 days after virus inoculation, observe the formation of plaques under a microscope, pick plaques, and further purify plaques, and then purify twice in this way.
[0092] (2) Passage stability analysis of the virus
[0093] Taking Vero cells as an example, the EV71 virus strain was selected for continuous passage on Vero cells, harvested when the lesion was 75% to 90%, and the tite...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com