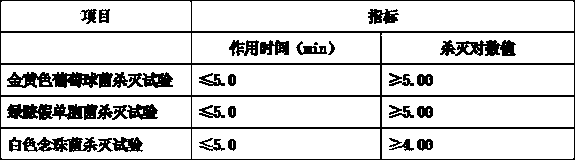
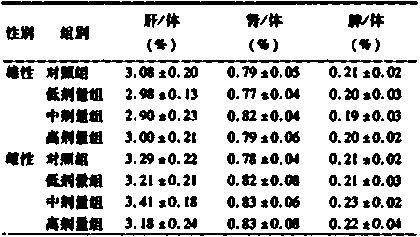
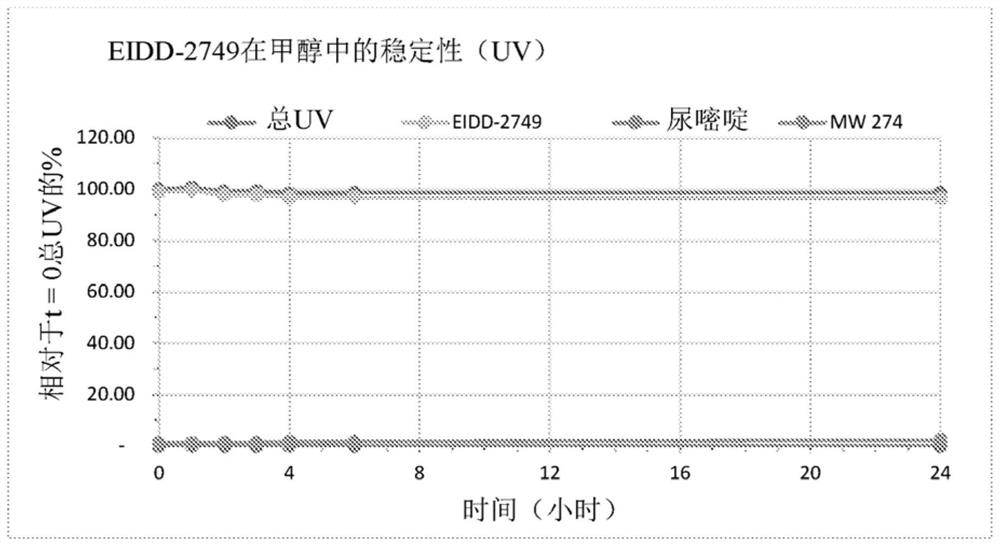
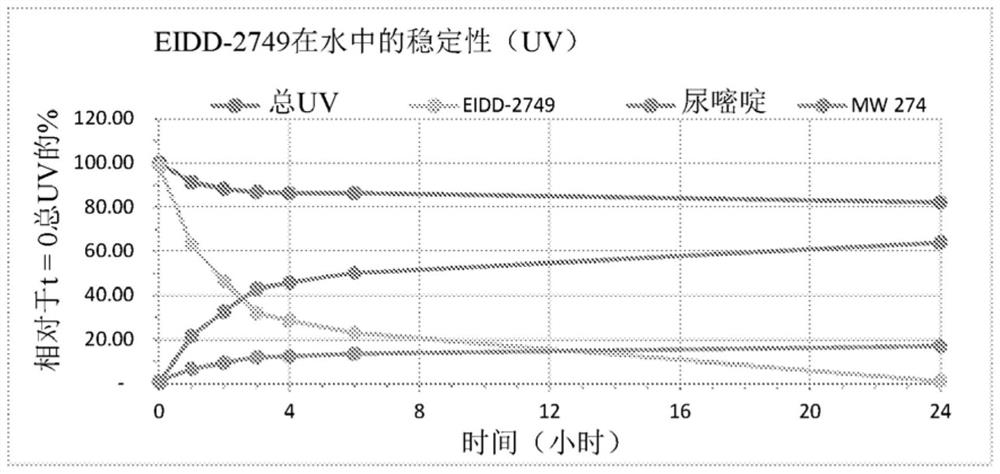
Patents
Literature
179 results about "Viroid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viroids are the smallest infectious pathogens known. They are composed solely of a short strand of circular, single-stranded RNA that has no protein coating. All known viroids are inhabitants of higher plants, in which most cause diseases, ranging in economic importance.
Purification of adenovirus and AAV
InactiveUS7015026B2Rapidly and efficiently purifyHigh molecular weightEnzymologyRecovery/purificationBiomedical engineeringGenetic transfer
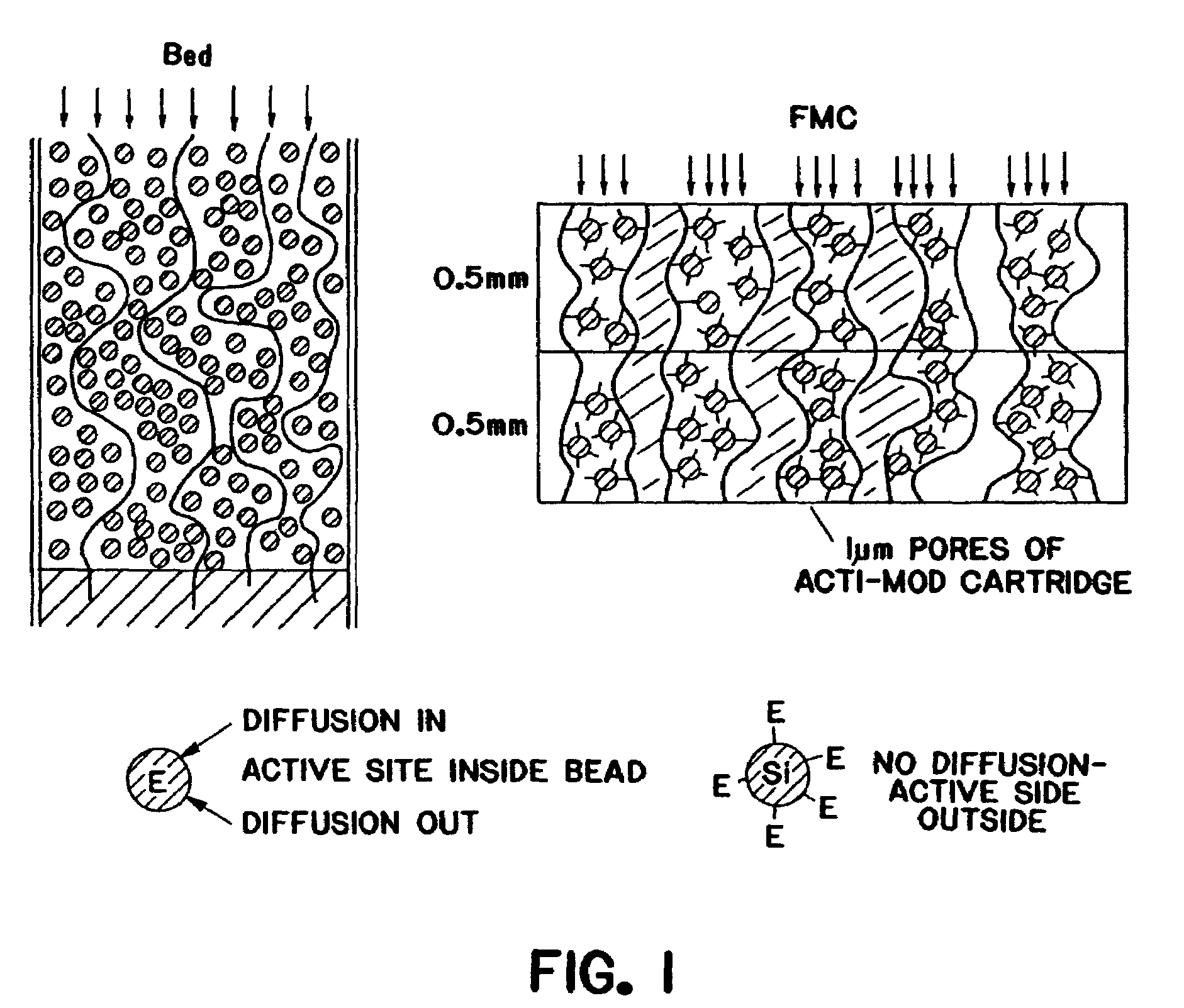
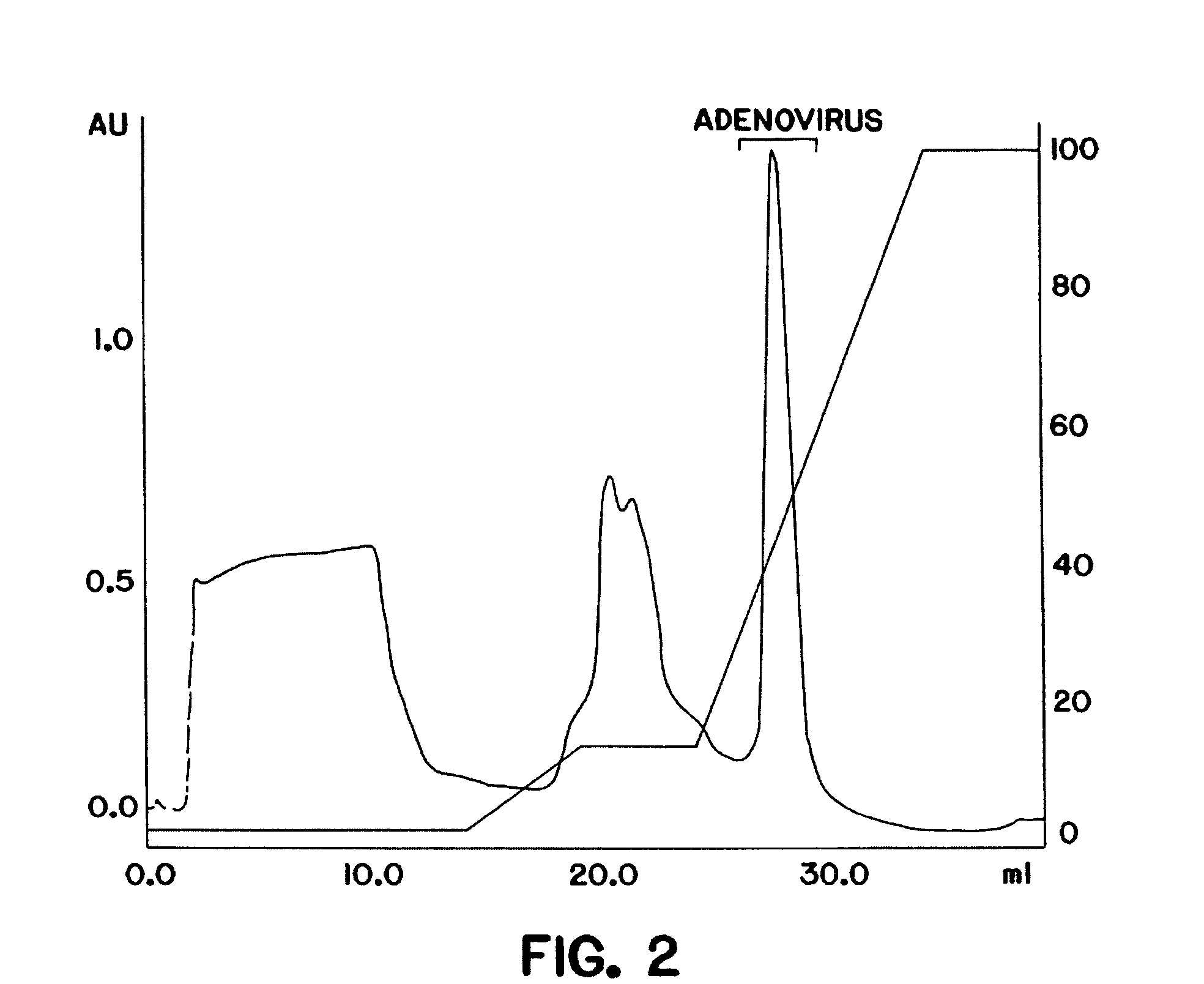
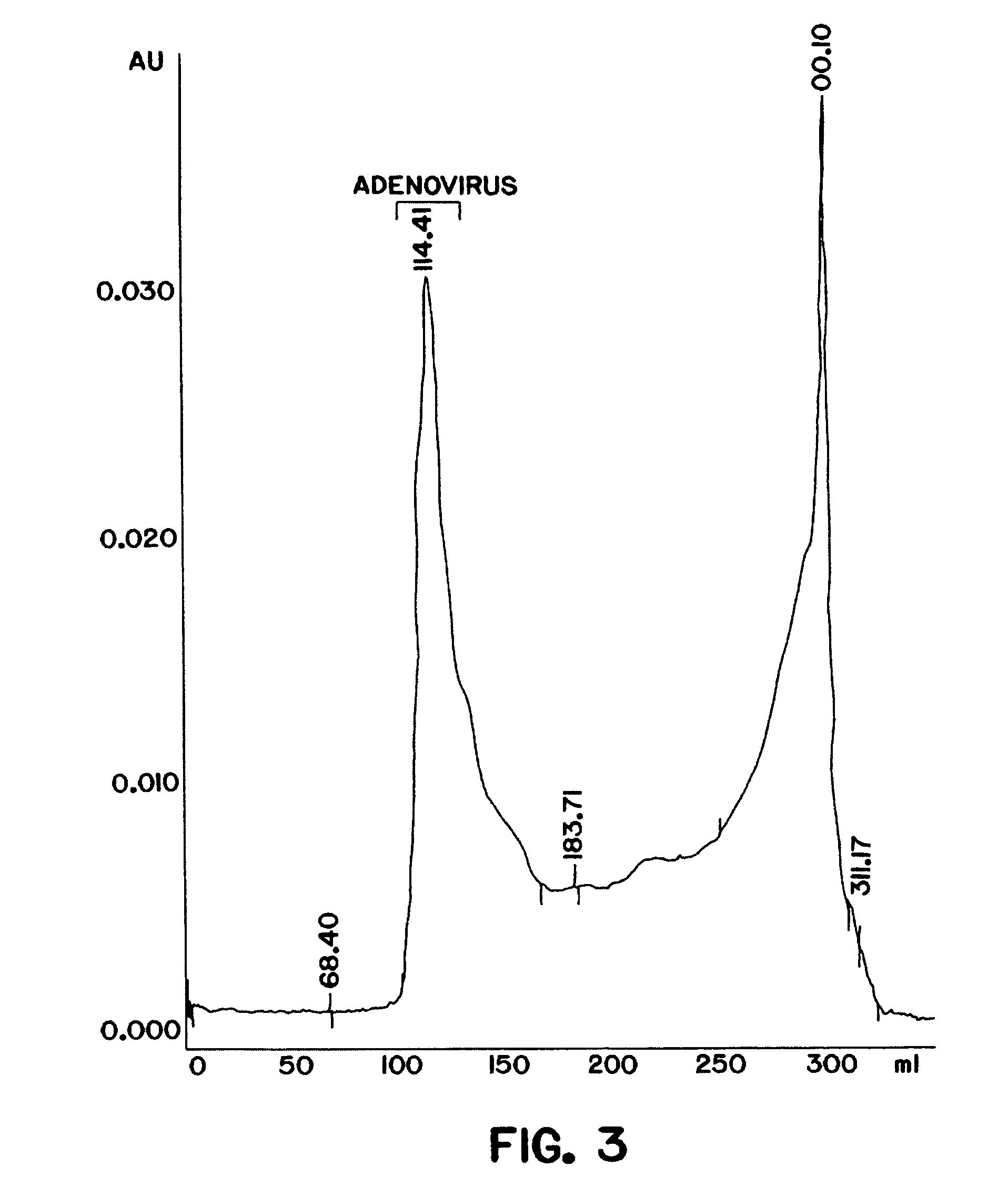
The present invention relates to the purification of large scale quantities of active (infectious) adenovirus and AAV, especially for use in therapeutic applications. In particular, the invention provides improved methods for contacting such viruses with suitable chromatographic materials in a fashion such that any damage to the virus, particularly to surface components thereof, resulting from contact with such chromatographic materials is minimized or eliminated. The result is the ability to rapidly and efficiently purify commercial level quantities of active (infectious) virus suitable for use in therapeutic applications, e.g. gene transfer / therapy procedures.
Owner:GENZYME CORP
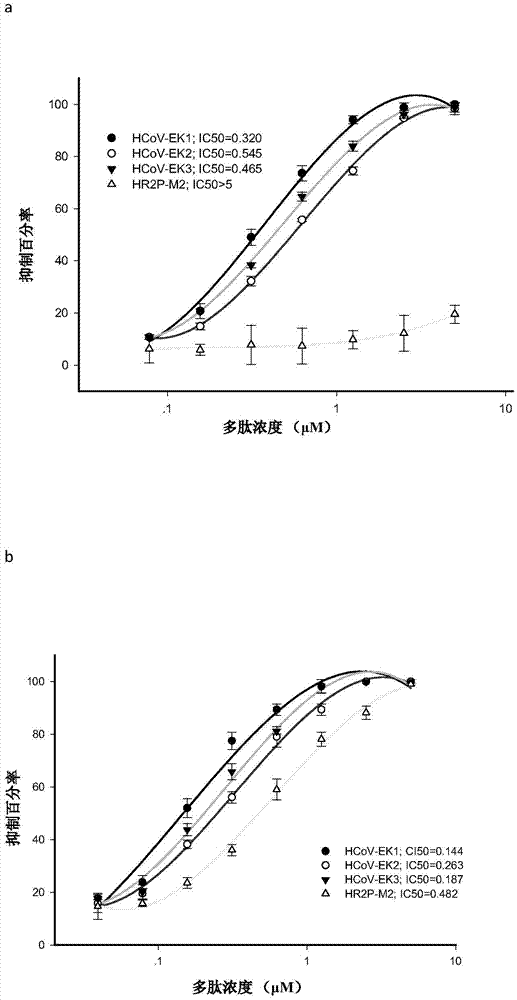
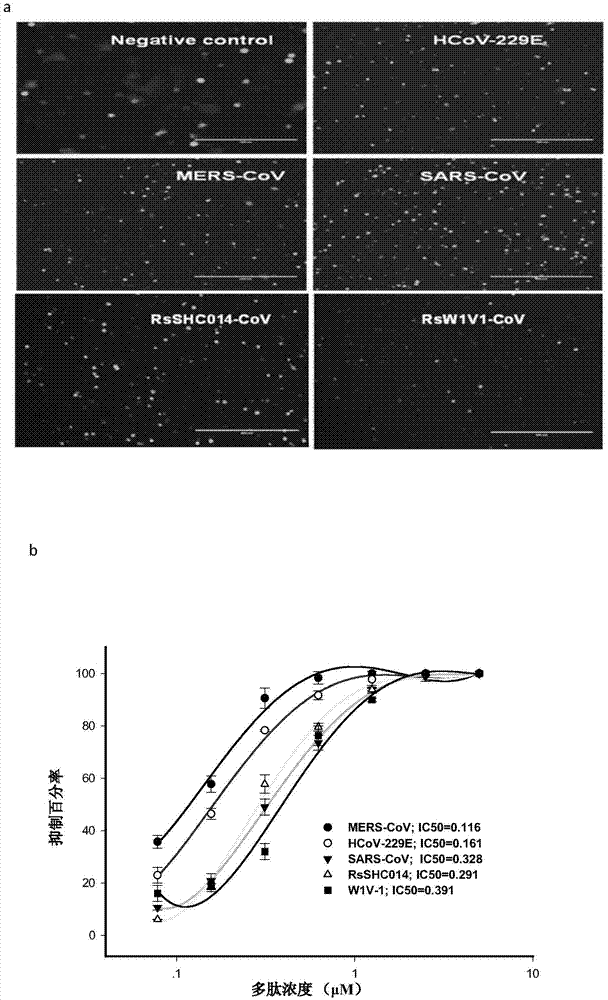
Polypeptides capable of inhibiting human coronavirus infections in a broad spectrum manner, and applications thereof
ActiveCN107022008AEnhanced inhibitory effectStrong inhibitory activitySsRNA viruses positive-sensePeptide/protein ingredientsHuman coronavirusFusion mechanism
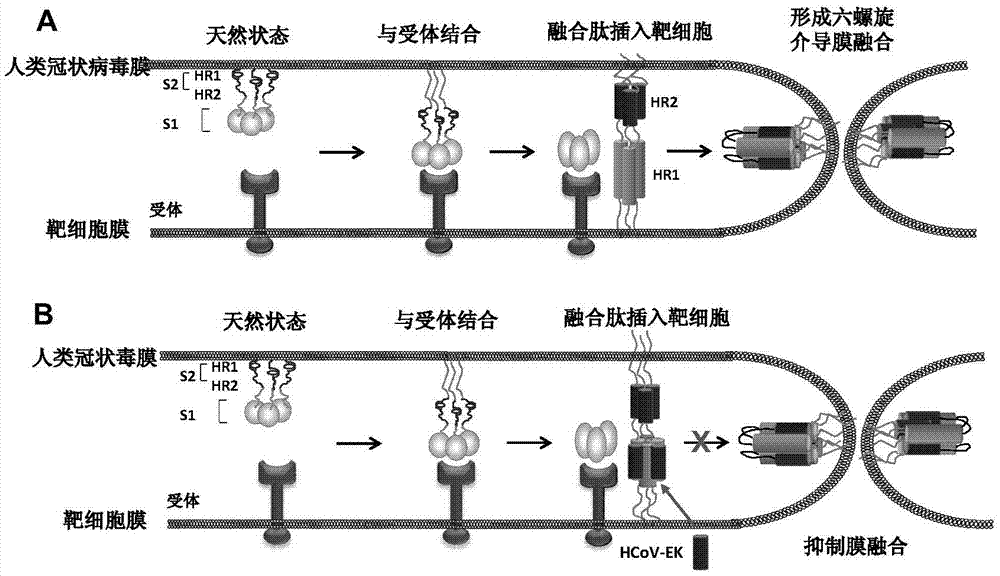
The present invention belongs to the field of biomedicine, and relates to polypeptides capable of inhibiting human coronavirus infections, particularly to polypeptides capable of inhibiting human coronavirus infections in a broad spectrum manner, and applications thereof. According to the present invention, the polypeptides capable of providing broad spectrum inhibition effects for infections caused by more than 2 human coronaviruses are provided based on the conservation property of the S2 region of a coronavirus S protein and the similar fusion mechanism; the test results show that the polypeptides can achieve the commonality of the human coronavirus, ie., the similar HR region and the same fusion mechanism mediated by the coronavirus S protein so as to provide a series of HCoV-EK polypeptides, wherein the polypeptides provide good inhibition effects for currently popular human coronaviruses, and further provide good inhibition activity for SARS virus (RsSHC014-CoV or RsW1V1-CoV) possibly infecting human; and the polypeptides of the present invention can provide the prevention and treatment candidate drug for the currently popular human coronaviruses and the novel human coronavirus possibly emerging in the future.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Gene chip for detecting plant virus
InactiveCN1600865AImprove the detection rateHigh outputMicrobiological testing/measurementGeneViroid
This invention relates to a gene-chip for plant virus detection, particularly it relates to a method, by utilizing gene-chip, to detct samples if it contains plant virus. It is used for novel nucleic acid primer of PCR, or, for detecting PCR product probe. This method can be used for detecting simultaneously determinand sample with 2-122 kinds of virus, sich as: AIMV, CMV, PVA, PVX, PVY, TMY, BBTV, LSV, SqMV, CEVD, ASGV, PXP, PVS, LMoV, DsMV, ZYMV, WMV, CRSV, CCCVd, PNRSV, SBMV, TRSV and viroid PSTVd etc.
Owner:北京金长河科技发展有限公司
Shorten human papilloma virus 16 type L1 protein
The invention relates to a truncated human papillomavirus type 16 L1 protein, virus-like particles composed of the protein, a vaccine containing the virus-like particles, and an application thereof in preventing cervical cancer.
Owner:XIAMEN INNOVAX BIOTECH +1
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Aav vector and assay for Anti-aav (adeno-associated virus) neutralizing antibodies
ActiveUS20160123990A1Microbiological testing/measurementGenetic material ingredientsAnti virusViral antibody
Virus vectors, virus particles, and methods and uses of screening for, detecting, analyzing and determining amounts of virus antibody, or neutralizing antibody activity of samples are provided. Such virus vectors, virus particles, and methods and uses are applicable to a broad range of virus types, such as lentiviruses, adenovirus, and adeno-associated virus (AAV) serotypes. Methods and uses include virus antibody screening, such as anti-virus immunoglobulins screened for, detected, analyzed and amounts determined
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
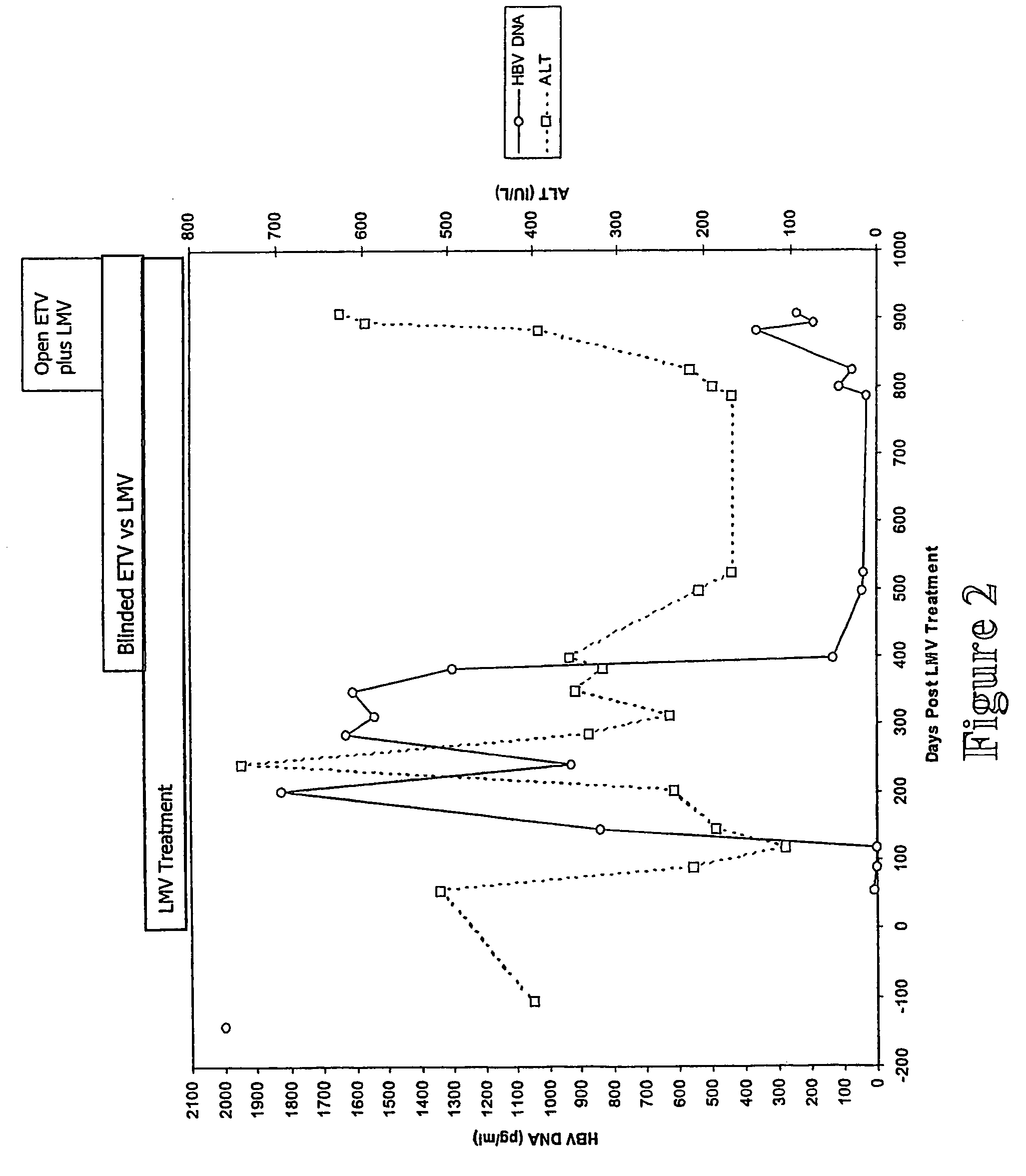
Viral variants with altered susceptibility to nucleoside analogs and uses thereof
The present invention relates generally to viral variants exhibiting reduced sensitivity to particular agents and / or reduced interactivity with immunological reagents. More particularly, the present invention is directed to hepatitis B virus (HBV) variants exhibiting complete or partial resistance to nucleoside analogs and / or reduced interactivity with antibodies to viral surface components including reduced sensitivity to these antibodies. The present invention further contemplates assays for detecting such viral variants, which assays are useful in monitoring anti-viral therapeutic regimens and in developing new or modified vaccines directed against viral agents and in particular HBV variants. The present invention also contemplates the use of the viral variants to screen for agents capable of inhibiting infection, replication and / or release of the virus.
Owner:ABL SA
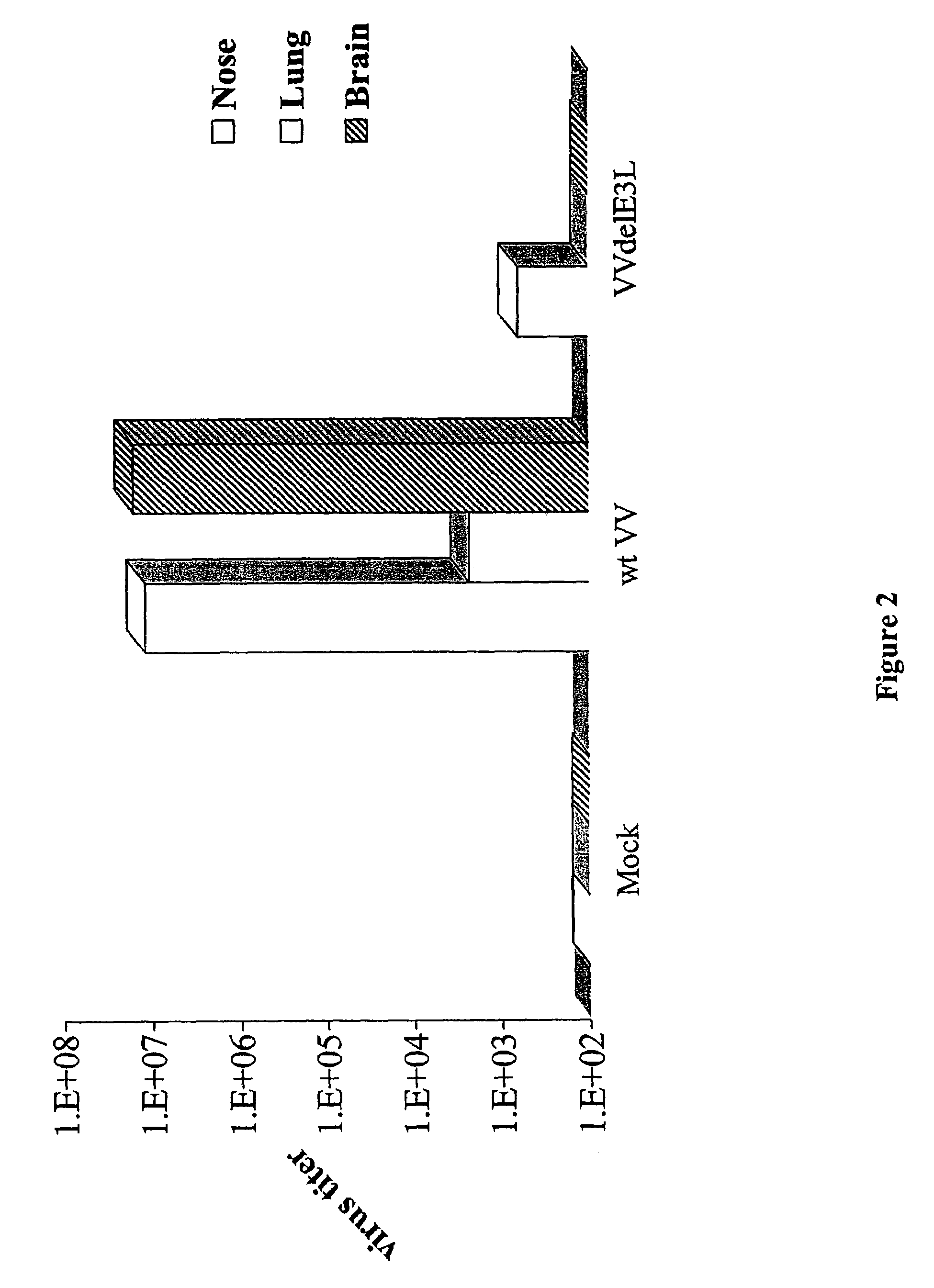
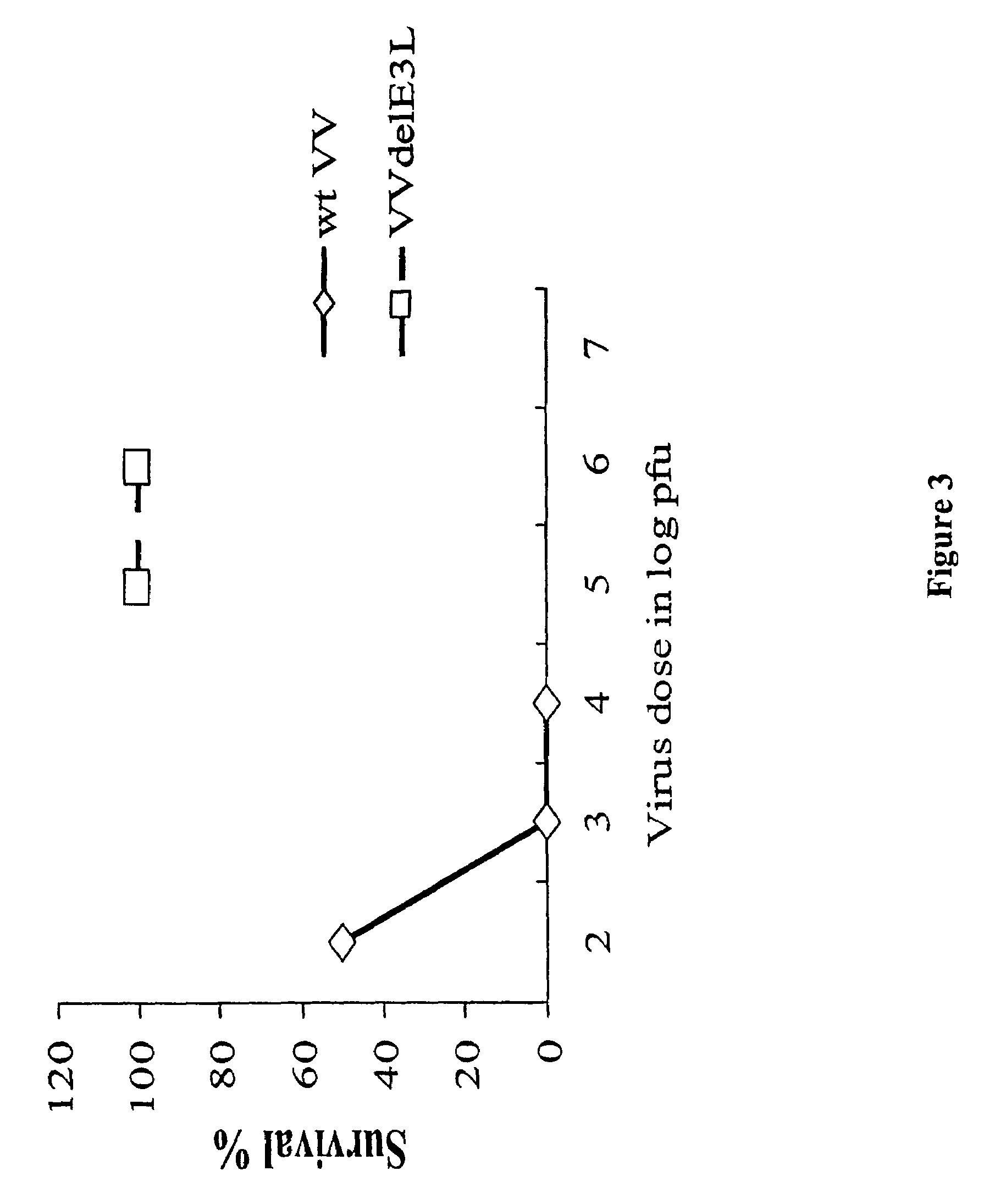
Use of vaccinia virus deleted for the E3L gene as a vaccine vector
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses containing an inactivated E3L region. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The invention is based on the discovery that vaccinia virus mutants having deletions in the E3L region exhibit dramatically reduced pathogenesis while remaining highly immunogenic. In addition, the invention relates to modified recombinant vaccinia viruses engineered to express heterologous polypeptides and the use of such viruses in vaccines designed to stimulate a protective immune response against such polypeptides in a host. The invention further relates to an interferon-sensitive recombinant vaccinia virus with broad host range wherein a salamander eIF2α is inserted into the viral genome in place of at least a portion of the E3L gene.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Truncated human papilloma virus 18 type L1 protein
The invention relates to a truncated human papilloma virus 18 type L1 protein, and viruslike particles consisting of the truncated human papilloma virus 18 type L1 protein, vaccine containing the viruslike particles and the application thereof in preventing cervical cancer.
Owner:XIAMEN INNOVAX BIOTECH +1
cDNA corresponding to the antigenome of nonsegmented negative strand RNA viruses and process for the production of such viruses
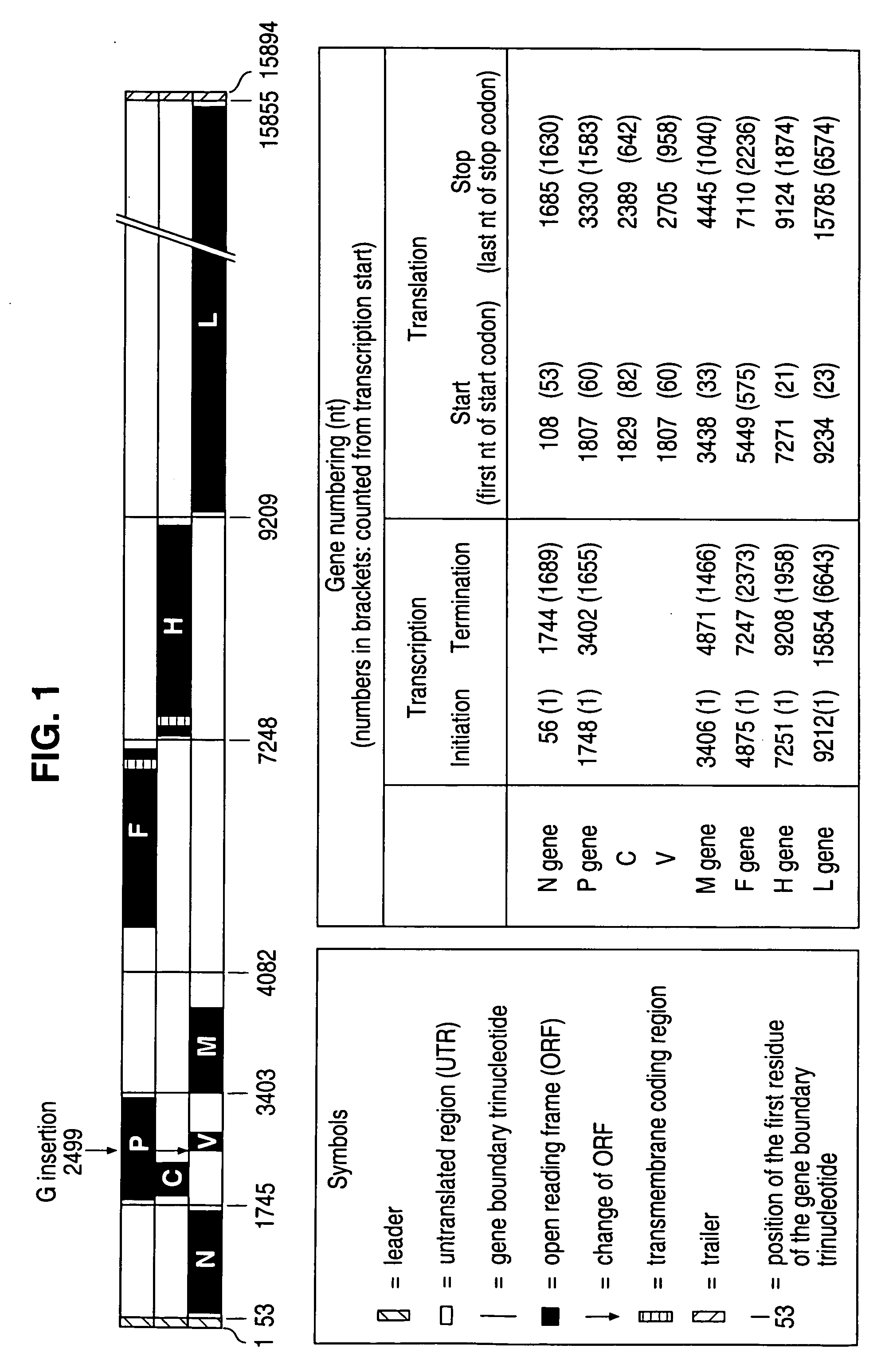
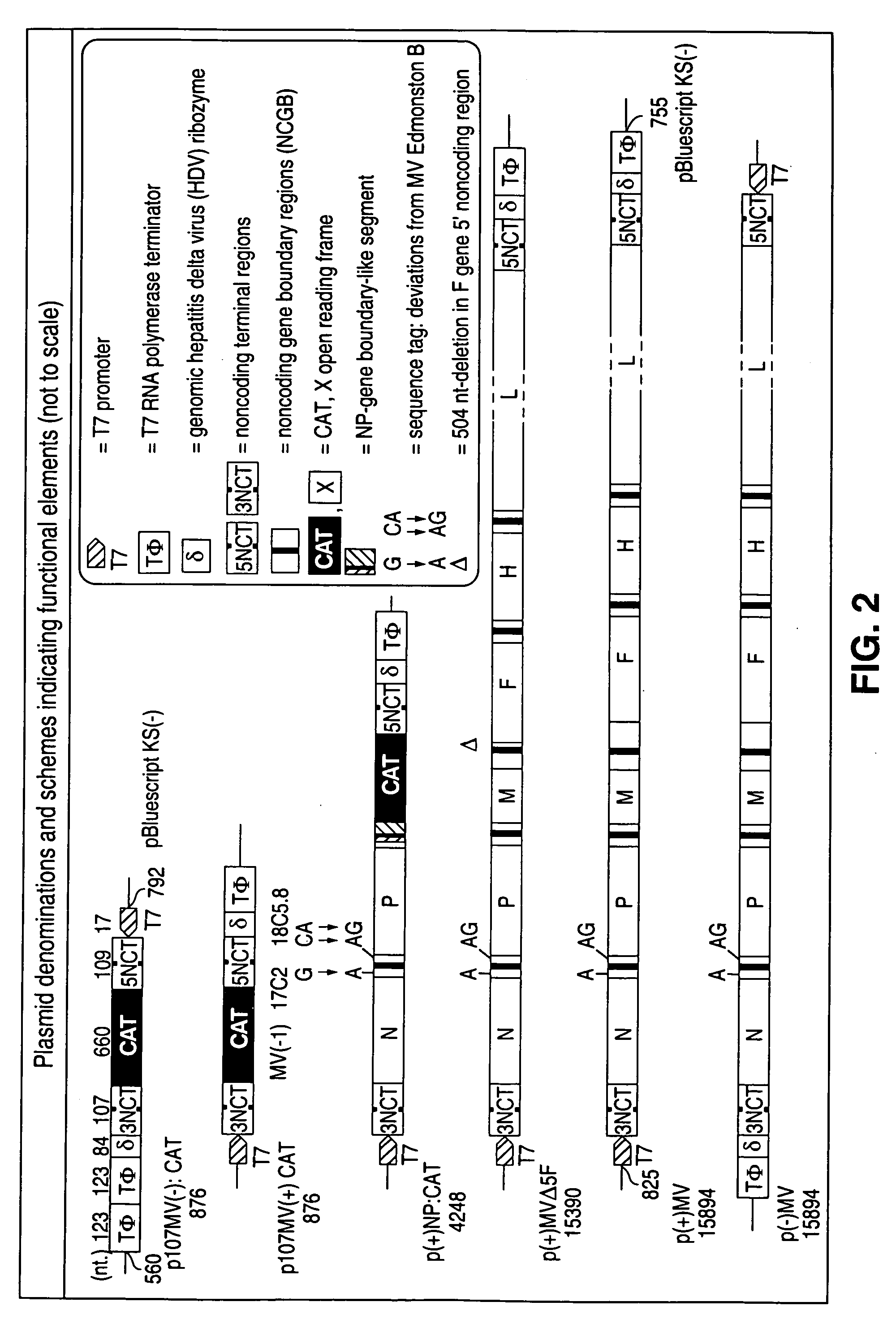
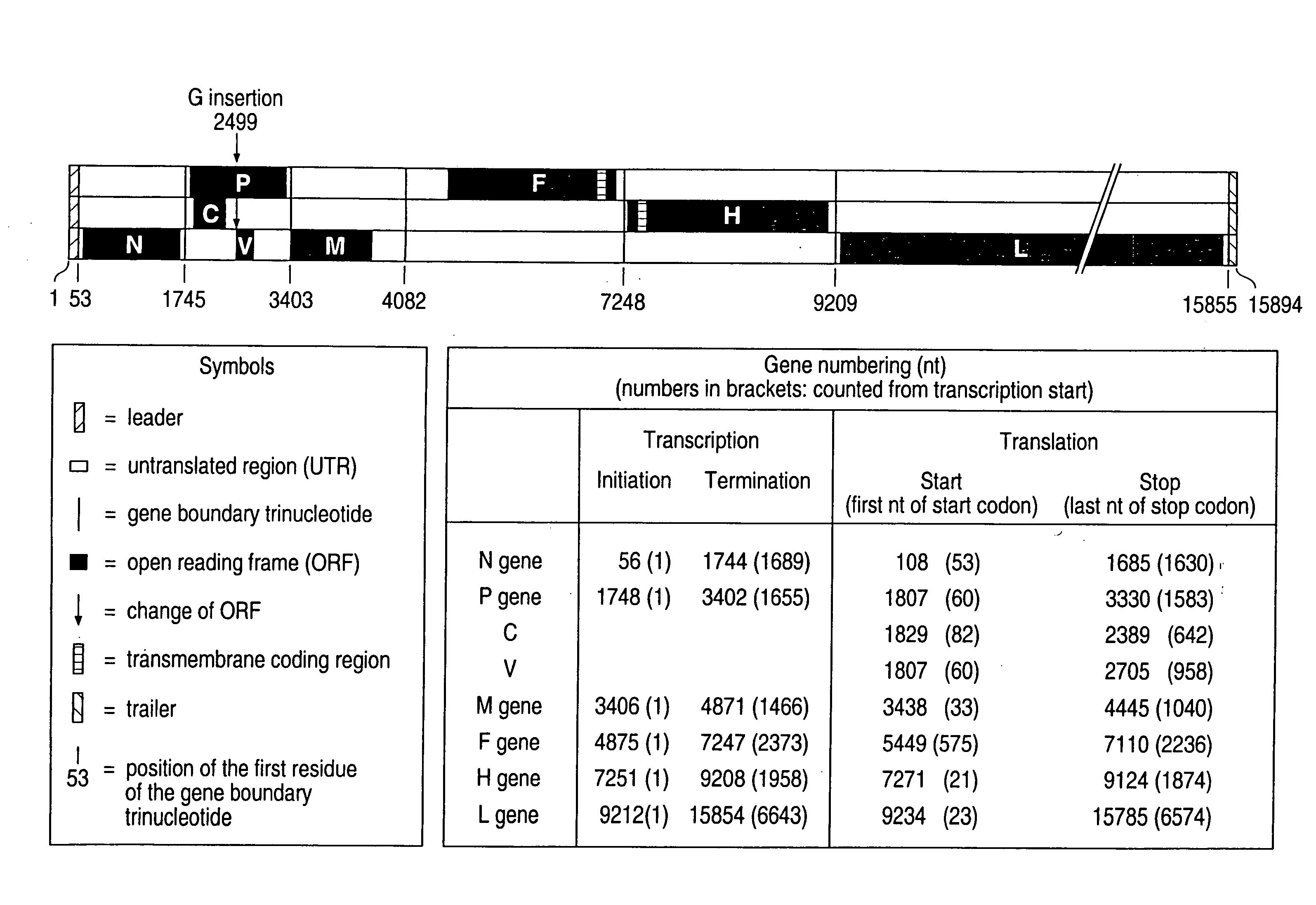
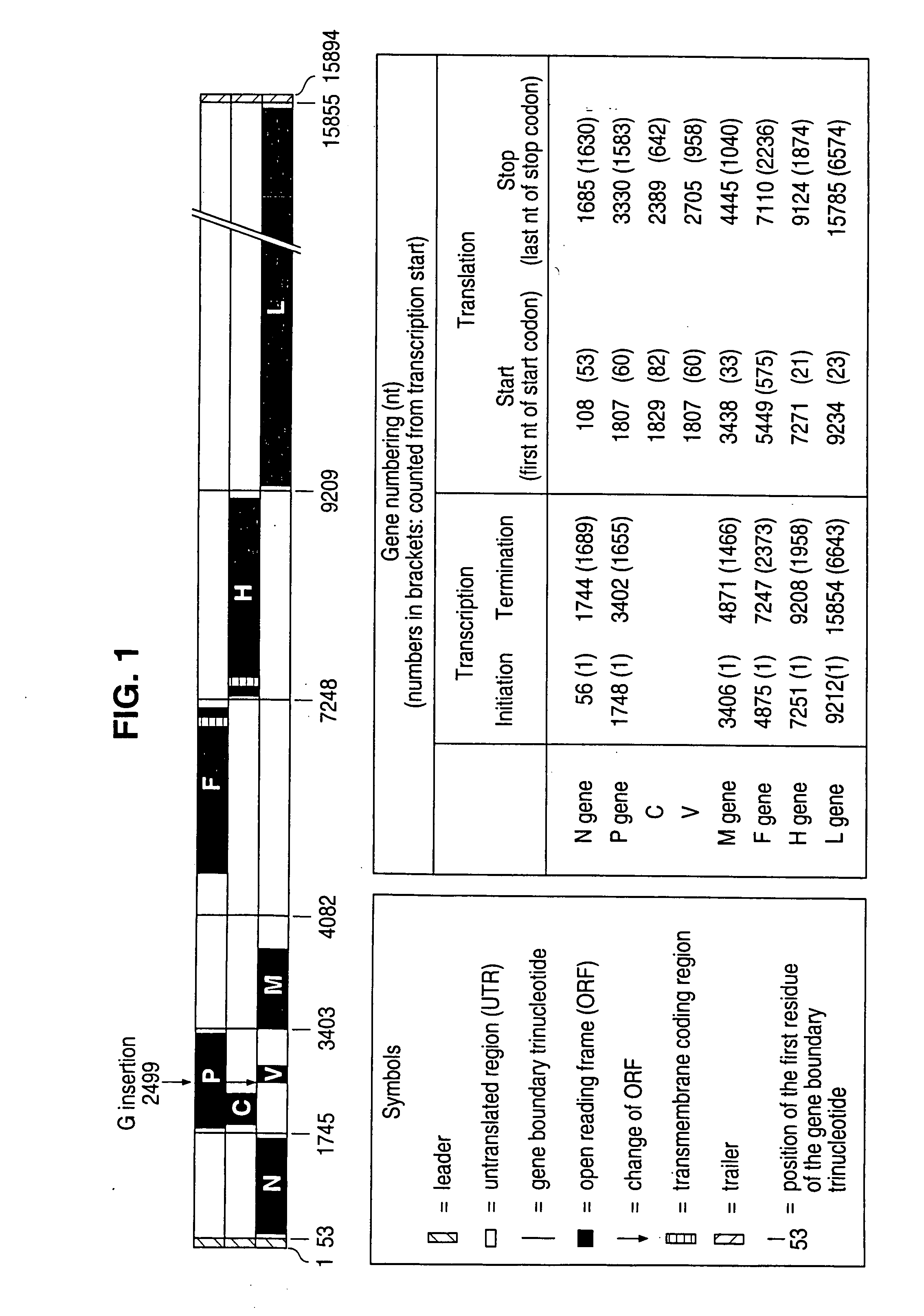
The present invention relates, in general, to a methodology or the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a model for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
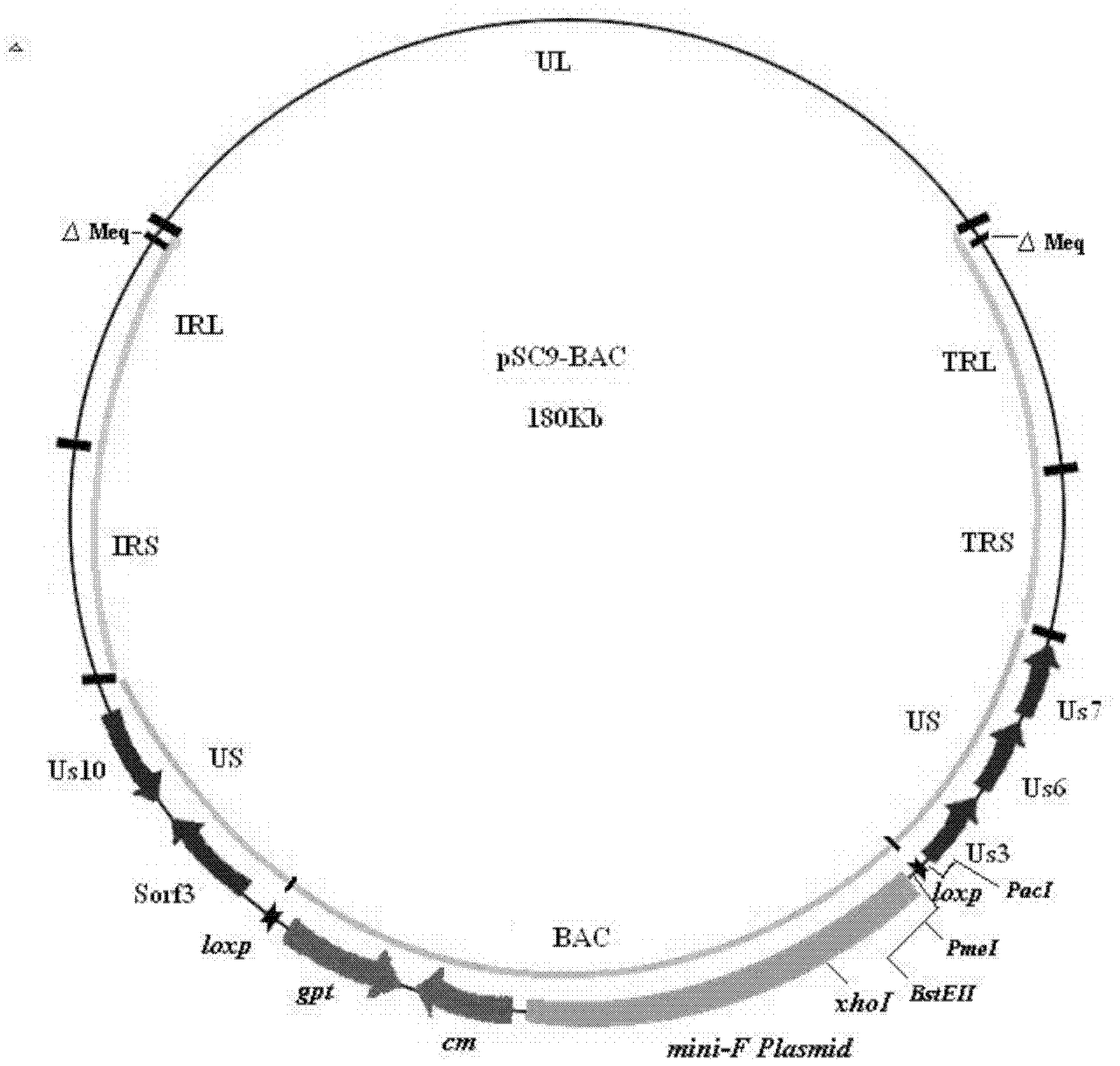
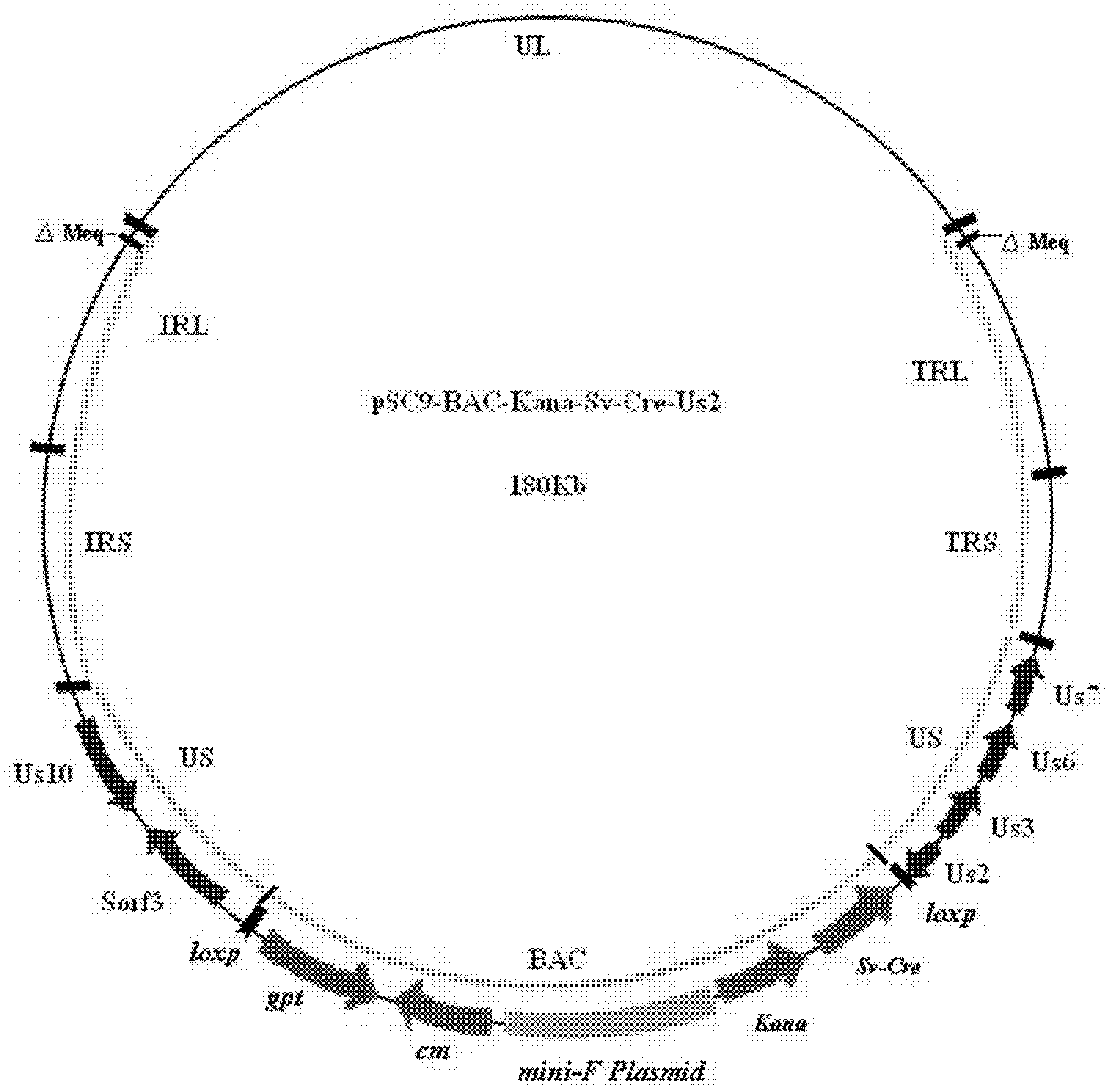
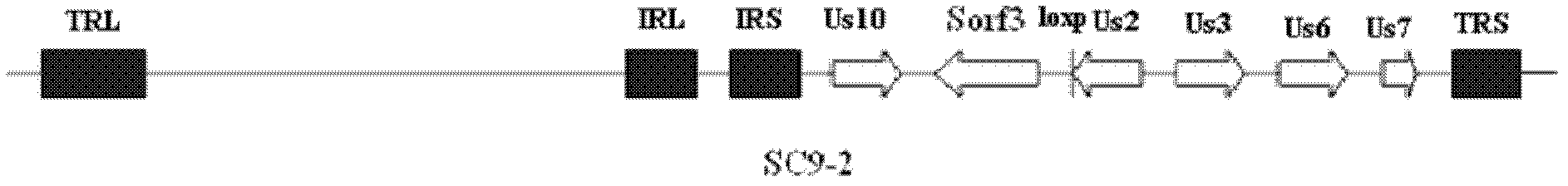
Construction and application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain
The invention relates to a construction and an application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain. The recombinant virus construction method solves the technical problem that kanr gene cannot be knocked out again by a present method after kanr gene containing flp recognition sites at two ends is continuously used twice to knock out two specific functional genes on the same virus genome. The obtained recombinant virus MDV SC 9-1 strain and MDV SC 9-2 strain are used as production strains of Marek's Disease live vaccine. The prepared vaccine prevents the very virulent or emerging very virulent plus MDV induced chicken Marek's disease. The protective immunity effect of the vaccine is superior to CVI988 / Rispens strain vaccine which is mostly widely used in foreign and domestic markets at present. The antigenicity of the recombinant virus is more similar to that of Chinese epidemic strain than the antigenicity of the similar virus rMd5deltameq which has been published in the United State. The strains provided by the invention will not induce tumor and has no immune suppression effect. Therefore, the strains are more applicable to China.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
cDNA Corresponding to the antigenome of nonsegmented negative stranded RNA viruses and process for the production of such viruses
The present invention relates, in general, to a methodology for the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a mode for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
Primer probe for identifying novel coronavirus and application of primer probe in triple fluorescent RPA
ActiveCN111235316ALow detection sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acid detectionViral nucleic acid
The invention discloses a triple RPA primer, probe, identification method and identification kit for rapidly screening and detecting a novel coronavirus (SARS-CoV2) and other SARS-similar coronaviruses. A first set of primers and probes (marked as FAM) are designed in a region of an N gene sequence of the SARS-CoV2 virus; a second set of primers and probes (marked as HEX) are designed in an E genesequence in a conserved region of an SARS-similar virus; and a third set of primers and probes (marked as Cy5, being different from the other SARS-similar coronaviruses) are designed in a specific region of an S gene sequence of the SARS-CoV2 virus. Compared with qPCR nucleic acid detection methods recommended, by the WHO and the national CDC, to be used in novel coronavirus clinics, the triple RPA primer, the probe, the identification method and the identification kit have the advantages that the sensitivity is high, the detection speed is high, and the novel coronavirus and the other SARS-similar coronaviruses can be effectively distinguished; and the nucleic acid detection time limit of the virus can be shortened from 90 minutes to 20 minutes, and rapid screening demands on the novel coronavirus and the other SARS-similar coronaviruses can be satisfied.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Degradable liquid mulching film and preparation method thereof
InactiveCN110423380AReduce dosageEasy to degradePlant protective coveringsPolyvinyl alcoholPlastic mulch
The invention discloses a degradable liquid mulching film and a preparation method thereof. The degradable liquid mulching film comprises the following raw materials by weight: 0.5-1.5 parts of polyvinyl alcohol, 1.5-4.5 parts of carboxymethylcellulose, 0.3-0.9 part of chitosan, 0.2-0.6 part of gelatin, and 0.05-0.10 part of humic acid. The degradable liquid mulching film has the characteristics of simple preparation method, low cost and convenient use, strong water absorption and water retention capacity, easily degradable components and no residue. Film mulching and cemented soil grains areutilized to reduce the evaporation of soil water, reduce the salt content of a plow layer, improve the physical and chemical properties of soil, and increase the soil temperature. The degradable liquid mulching film also has bactericidal effect and the capability of inhibiting plant viruses and viroids, and protects plants from the infection of bacteria and viruses, at the same time can promote the proliferation of beneficial bacteria in soil and obviously improve microfloras.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Cold-adapted equine influenza viruses
InactiveUS20060121521A1Antibacterial agentsSsRNA viruses negative-senseVirus influenzaRespiratory disease
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus or other infectious agents utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to an infectious agent, as well as using a therapeutic composition as a treatment for an animal that has been recently infected with an infectious agent leading to respiratory disease, or is likely to be subsequently exposed to such an agent in a few days whereby the therapeutic composition reduces such respiratory disease, even in the absence of antibody-mediated immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaptation.
Owner:DOWLING PATRICIA W +1
Method for purifying prokaryotic cell expressed viroid particle
ActiveCN104673760AHigh recovery rateShort reaction timeInactivation/attenuationPeptide preparation methodsEscherichia coliPurification methods
The invention relates to a method for purifying an escherichia coli expressed viroid particle. The purification method comprises a precipitation step, a first chromatography step, a second chromatography step, a concentration step and a third chromatography step, wherein the precipitation step comprises an ammonium sulfate precipitation step; the first chromatography step comprises a molecular screen chromatography step; the second chromatography step comprises a hydroxyapatite chromatographic analysis; the concentration step comprises an ultrafiltration and concentration step; the third chromatography step comprises a heparin affinity chromatography step.
Owner:JIANGSU THERAVAC BIO PHARMA
Treatment of neoplasms with viruses
InactiveUS20080057037A1Convenient treatmentSsRNA viruses negative-senseBiocideDiseaseAnti viral response
The subject invention relates to viruses that are able to replicate and thereby kill neoplastic cells with a deficiency in the IFN-mediated antiviral response, and their use in treating neoplastic disease including cancer and large tumors. RNA and DNA viruses are useful in this regard. The invention also relates to methods for the selection, design, purification and use of such viruses for cancer therapy.
Owner:WELLSTAT BIOLOGICS CORP
Bio-organic fertilizer and manufacturing method thereof
InactiveCN106083452AImproved soilTreatment of heavy metal and waste organic matter pollutionBio-organic fraction processingAnimal corpse fertilisersChemistryHeavy metals
The invention discloses bio-organic fertilizer and a manufacturing method thereof. The manufacturing method comprises mixing 60-80 parts by weight of animal feces, 20-40 parts by weight of plant organic waste residues, 1-3 parts by weight of probiotics, 0.5-2 parts by weight of a mixture of honey, brown sugar and milk and 0.5-2 parts by weight of a mixture of licorice root, rheum officinale and lumbricus, crushing the mixture, carrying out stacking fermentation, carrying out propagation, carrying out control drying, and fragmenting the fermentation product to obtain a mixture with a single particle size less than 10mm, wherein the mixture is the bio-organic fertilizer. The bio-organic fertilizer has obvious effects of preventing and treating plant virus-caused diseases such as citrus tristeza, ginger bacterial wilt and strawberry yellows and can improve soil quality, treat heavy metal and waste organic matter-caused pollution and recover a soil ecological environment.
Owner:徐建斌
Multiple-PCR (polymerase chain reaction) detection and warning method for sweet potato chlorotic stunt virus (SPCSV) and sweet potato twin virus (Sweepoviruses) in sweet potato roots
ActiveCN106755579AStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseasePlant disease
The invention discloses a multiple-PCR (polymerase chain reaction) detection and warning method for sweet potato chlorotic stunt virus (SPCSV) and sweet potato twin virus (Sweepoviruses) in sweet potato roots. For the two types of viruses of SPCSV and Sweepoviruses, specific primer pairs CP-F and CP-R and primer pairs of VF and VR are designed, the detection primers are good in specificity, and only a stripe of the size of specificity can be amplified in a targeted virus sample. The detection method for SPCSV and Sweepoviruses is established by the aid of multiple-PCR primers, primary PCR reaction can be passed, two kinds of sweet viruses of SPCSV and Sweepoviruses can be detected whether to exist in the sweet potato roots or not, and quality of potato seeds is further judged; relation of sweet potato root virus with sweet potato seedling, field period virus symptoms and yield loss is determined, basis is provided for judging whether the potato seeds are qualified or not by detecting whether the potato seeds carry the virus or not, and great importance is achieved in quality of detoxified sweet potatoes and disease epidemic warning.
Owner:INST OF PLANT PROTECTION HENAN ACAD OF AGRI SCI
ELISA-Array method for detection of encephalitis viruses, and special kit thereof
InactiveCN102426237AChemiluminescene/bioluminescenceAgainst vector-borne diseasesEncephalitis VirusesCapture antibody
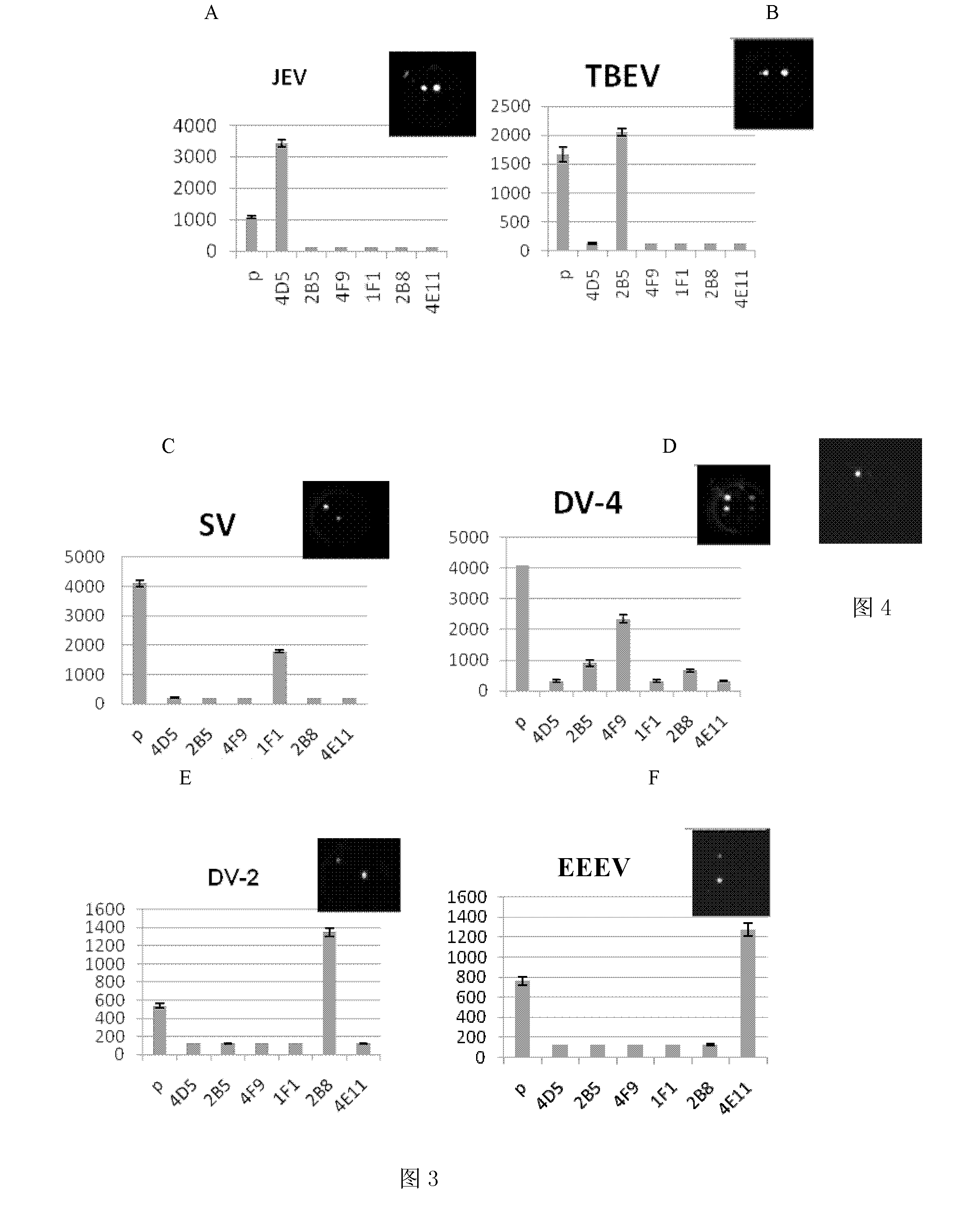
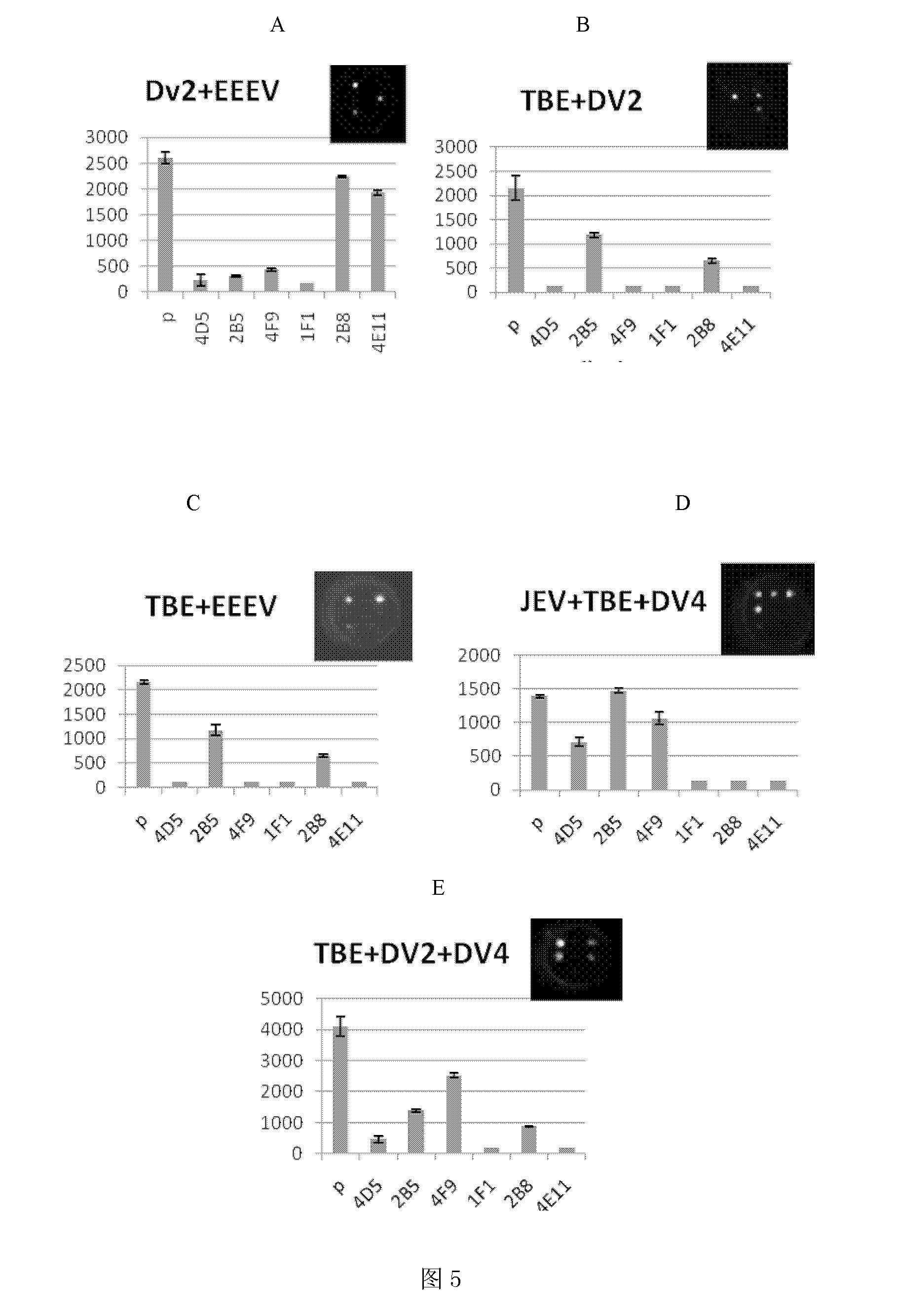
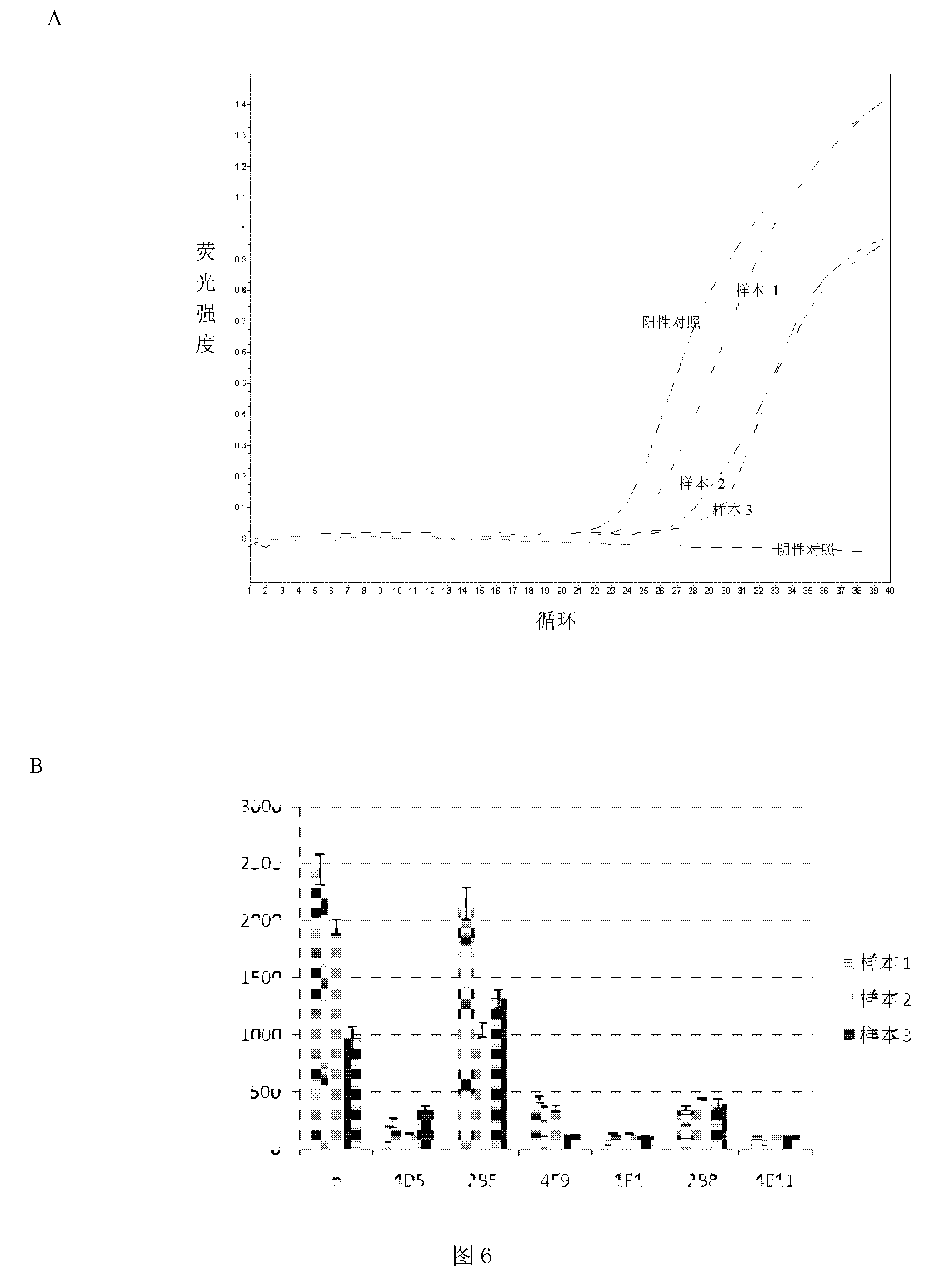
The present invention discloses an ELISA-Array method for detection of encephalitis viruses, and a special kit thereof. The kit of the present invention comprises six capture antibodies, wherein the solution of each capture antibody is a mixing solution prepared by mixing the capture antibody and a spotting solution, the concentration of each capture antibody in the corresponding capture antibody solution is 0.05 mg / ml. Experimental results of the present invention show that: the six specific encephalitis virus monoclonal antibodies are prepared by the method of the present invention; with adopting the ELISA-Array technology platform, and optimizing the experimental conditions, the ELISA-Array technology capable of concurrent detection of the six encephalitis viruses is established, and can be adopted for detection of the six encephalitis-related viruses; compared to the common ELISA, the ELISA-Array method of the present invention has the following advantages that: the specificity of the ELISA-Array method is the same as the specificity of the common ELISA, the sensitivity is high, the highest sensitivity is more than 10 times the sensitivity of the common ELISA, and the ELISA-Array method has a high clinical application prospect.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Bacteriostatic agent for sterilization and disinfection, skin mucosa disinfection fluid and preparation method thereof
The invention discloses a bacteriostatic agent for sterilization and disinfection, skin mucosa disinfection fluid and a preparation method thereof. The bacteriostatic agent is mainly prepared from honeysuckle, bo-chrysanthemum, mint, dandelion, folium isatidis and chlorhexidine acetate according to a certain weight ratio. The bacteriostatic agent is prepared by a basic formula of the Central Treasury Canon written by Huatuo according to a research and is a high-tech natural disinfection product refined by combining the modern traditional Chinese medicinal science practice and the modern traditional Chinese medicinal technology; the disinfection fluid and the bacteriostatic agent are prepared according to the reliable practice and the theory basis and can effectively kill various fungi, mildew, tritirachium album, staphylococcus aureus, escherichia coli, various types of viruses and conventional pathogenic bacteria.
Owner:ANHUI YIRENAN
Degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and kit for hantavirus group
InactiveCN102382907AStrong specificityIncreased sensitivityMicrobiological testing/measurementOligonucleotide primersInverse polymerase chain reaction
The invention discloses a degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and a kit for hantavirus group. The detection reagent comprises a pair of degenerate heterozygous oligonucleotide primers, and the sequences of the degenerate heterozygous oligonucleotide primers are respectively G1: GCAACAGCAACATGGTTTcartaytayac and G2: CTTCTTCATTCATATTTCCATGCarnccyttytc; the non-merger consensus sequence of the 5' ends in the primers plays a role in stabilizing the combination of a 3' merger core area and a template under the condition that the degeneracy of the primers is not increased, so that the specificity of the degenerate PCR reaction is improved, various viruses in hantavirus can be amplified and detected, the homologous unknown viruses of the extendedgenes can also be detected and the amplified target fragments can be sequenced by the detection reagent; and the kit are relatively high in sensitivity and good in hantavirus group specificity, can be used for detecting domestic popular Hantaan and Seoul viruses, and can also be used for detecting other oversea epidemic strains. The degenerate RT-PCR detection reagent and the kit for the hantavirus group are high in sensitivity, good in hantavirus specificity and universal.
Owner:中华人民共和国大榭出入境检验检疫局
4'-halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Disclosed are halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto. In certain embodiments, the disclosure relates to the treatment or prophylaxis of viral infections. Such viral infections can include tongaviridae, bunyaviridae, arenaviridae, coronaviridae, flaviviridae, picornaviridae, Eastern, Western, and Venezuelan Equine Encephalitis (EEE, WEE and VEE, respectively), Chikungunya fever (CHIK), Ebola, Influenza, RSV, and Zika virus infections.
Owner:EMORY UNIVERSITY
Purification of recombinant coxsackievirus A16 (CA16) virus-like particles, application thereof in vaccine and vaccine
ActiveCN109680026AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsPurification methodsUltrafiltration
The present invention provides a method for purifying recombinant coxsackievirus A16 (CA16) virus-like particles, an application thereof in an vaccine, and the vaccine. The method comprises carrying out high density fermentation culture of recombinantly expressed engineering bacteria and methanol-induced expression of CA16 viroid granulin, carrying out centrifugation for collecting thalli, performing high-pressure homogenization and breaking, and purifying the supernatant by ultrafiltration, ion exchange chromatography, hydroxyapatite chromatography and molecular sieve chromatography and the like. The CA16 viroid particle vaccine provided by the invention has good immunogenicity, safety, immunological characteristics and biological activity. The process is simple, and the purification is performed by chromatographic methods. The purification method is more conducive to linear amplification compared with density gradient centrifugation, and can be used in large-scale preparation and purification, and a high-purity (more than 99%) virus-like particle (VLP) stock solution can be obtained and can be used to prepare a vaccine for preventing CA16 infection, and has good economic value and application prospects.
Owner:深圳鑫泰康生物科技有限公司 +1
Construction method of Epinephelus fuscoguttatus heart cell line
InactiveCN101451121AIdeal for in vitro studiesSkeletal/connective tissue cellsInsulin-like growth factorLiquid medium
The invention relates to a method for constructing a heart cell system of blotchy rockcod, which comprises: taking a heart tissue of the blotchy rockcod as a material, adopting a trypsin digestion method to start primary culture, and culturing the heart tissue in a DMEM / F12 liquid medium which contains fetal calf serum, basic fibroblast growth factors, I-type insulin-like growth factors, chondroitin sulfate and culture supernatant of fin cells of the blotchy rockcod in the log phase and has a pH value of between 7.0 and 7.4; and adopting the trypsin digestion method for subculturing. The heart cell system of the blotchy rockcod constructed by the method is subcultured for 70 generations currently. The technology is scientific and reasonable, is hopeful to be applied to separation and breeding of fish viroids, preparation of virus vaccines and research in the aspects of interaction of viruses and host cells, and so on, can also be taken as a model for researching environmental pollutants in environmental toxicology, and performs pollution monitoring and safety evaluation on various environmental pollutants in the sea such as genic toxins, mutagen, carcinogens, environmental hormones and endocrine disruptors.
Owner:OCEAN UNIV OF CHINA
Biological preparation for preventing and controlling human respiratory syncytial virus infection and preparation method
ActiveCN104353063AImprove stabilityLow costPeptide/protein ingredientsAntiviralsHuman respiratory virusViral infection
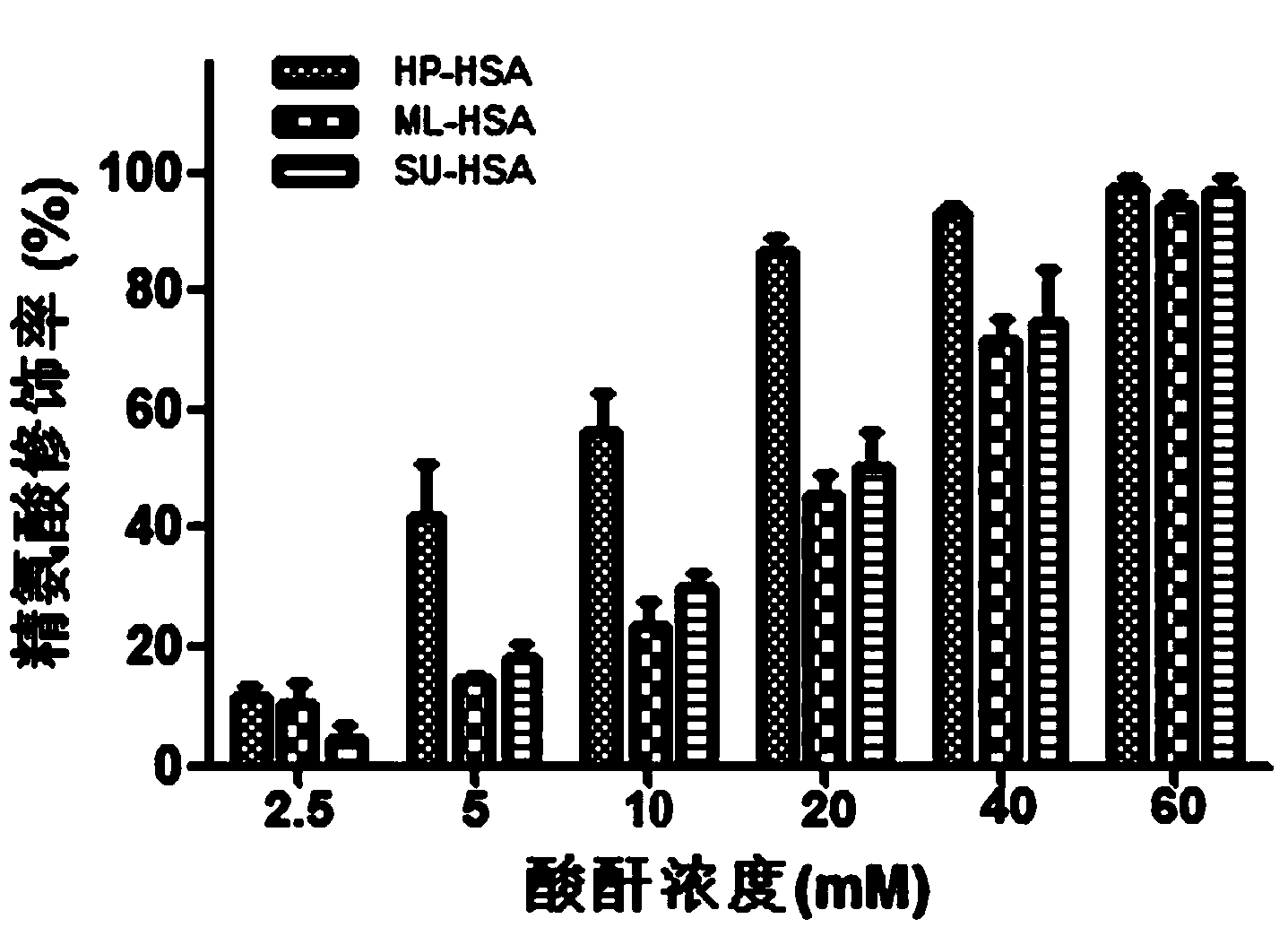
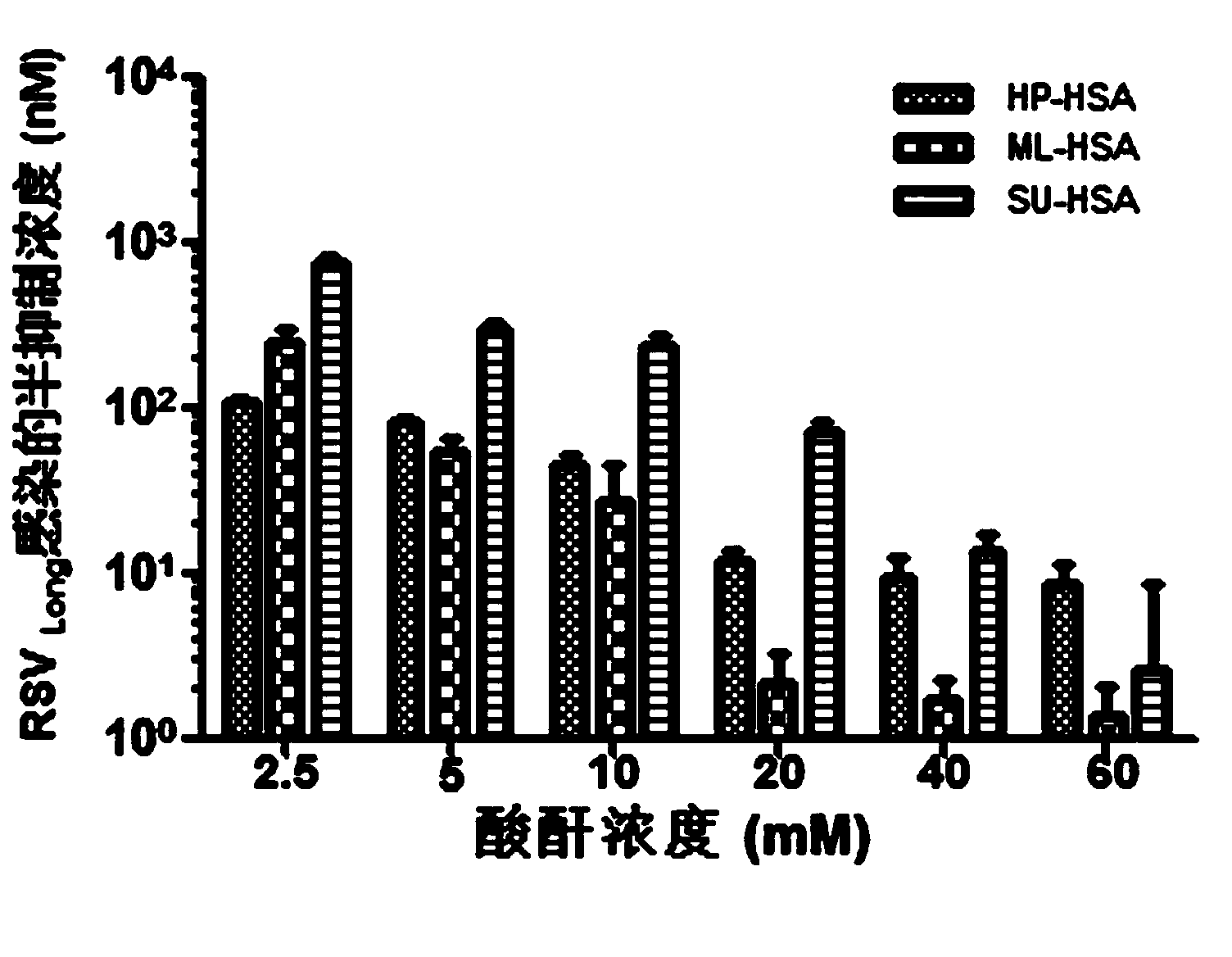
The invention relates to a novel antiviral biological preparation in the technical field of medicines, in particular to a biological preparation for preventing and controlling human respiratory syncytial virus infection and a preparation method. The biological preparation adopts globulin virus entry or fusion inhibitors. The biological preparation is applied to the first stage that virus invades target cells, and blocks the infection of virus to the cells, so as to achieve the effect of preventing and controlling the virus. The biological preparation adopts active anhydride to modify separated and purified amino acid with positive charge on the surface, thereby having the function of preventing the human RSV (respiratory syncytial virus) from entering and infecting the target cells.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Anti-fish viral haemorrhagic septicemia virus single-chain antibody
The invention discloses an anti-fish viral haemorrhagic septicemia virus single-chain antibody, a preparation method and application thereof, a gene for coding the single-chain antibody, and a vector, host cell and the like containing the gene. The anti-fish viral haemorrhagic septicemia virus single-chain antibody is formed by connecting an antibody heavy chain variable region and a light chain variable region through a joint peptide, and can be efficiently expressed through a prokaryotic expression system. The molecular weight of the single-chain antibody is 28kD or so; and the single-chain antibody can specifically identify the fish viral haemorrhagic septicemia virus and intercept the combination between the virus and natural serum, and can be further used for diagnosis of the virus, development of the therapeutic preparation and research of the antigen epitope.
Owner:OCEAN UNIV OF CHINA
Preparation method of nano-aluminium adjuvant capable of raising vaccine effect and its use
InactiveCN1824305ARapid activation of the immune responseReduce inflammationAntiviralsAntibody medical ingredientsDispersityAdjuvant
A nano-class aluminum adjuvant for improving the effect of vaccine is prepared from the aluminum hydroxide nanoparticles. It has high purity and dispersity. Said aluminum adjuvant is suitable for the substances prepared from virus or viroid component (protein, DNA, or RNA). Its preparing process is also disclosed.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Radix sophorae flavescentis and vinegar mixture for preventing and treating crop diseases
ActiveCN103988854AImprove immunityAchieve quality improvementBiocidePest repellentsAdjuvantBacterial disease
The invention discloses a radix sophorae flavescentis and vinegar mixture for preventing and treating crop diseases. The mixture comprises a main agent and selectively added auxiliary agents, wherein the main agent is prepared from the radix sophorae flavescentis and fermented vinegar which are immersed for 30-40 days according to the weight ratio of 1:10-15, the immersing temperature is 4-30 DEG C, the radix sophorae flavescentis and the fermented vinegar are evenly mixed to prepare the main agent of the radix sophorae flavescentis and vinegar mixture, or the main agent is prepared by a decocting method; the auxiliary agents are prepared by organosilicone adjuvants, cell membrane stabilizers, folium isatidis decoction and water; the auxiliary agents and the main agent are combined to prepare different radix sophorae flavescentis and vinegar compositions, and one or a combination of more than one of the different radix sophorae flavescentis and vinegar compositions are used to form the radix sophorae flavescentis and vinegar mixture. Chinese herbal medicines, auxiliary agents and adjuvants, which have the function of inhibiting the growth and the development of fungi, bacteria, viruses, mycoplasma, chlamydia, viroid and other pests, are selected to be prepared scientifically; the mixture prevents and treats bacterial diseases, fungal diseases and viral diseases of crops and pests of trialeurodes vaporariorum, aphid, thrips and the like; the mixed formula can be used for increasing the crop immunity, and the purposes of healthy cultivation, disease prevention and pest control, quality improvement and efficiency improvement for plants are achieved.
Owner:李春德
Process for expelling chrysanthemum flower viroid by cryogenic processing
InactiveCN1926965AEfficient removalSimple processing methodHorticulture methodsPlant tissue cultureChrysanthemum FlowerLow temperature treatment
The invention relates to a method for using low-temperature treatment to remove chrysanthemum virus. Wherein, it comprises low-temperature treatment, disinfection, cutting off and cultivating stem sharp, viroid detecting, cultivating neuter chrysanthemum. The invention can effectively remove chrysanthemum virus, with simple process and better effect.
Owner:河北省农林科学院经济作物研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com