Anti-fish viral haemorrhagic septicemia virus single-chain antibody
A technology of viral hemorrhage and single-chain antibody, which is applied in antiviral agents, antiviral immunoglobulins, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
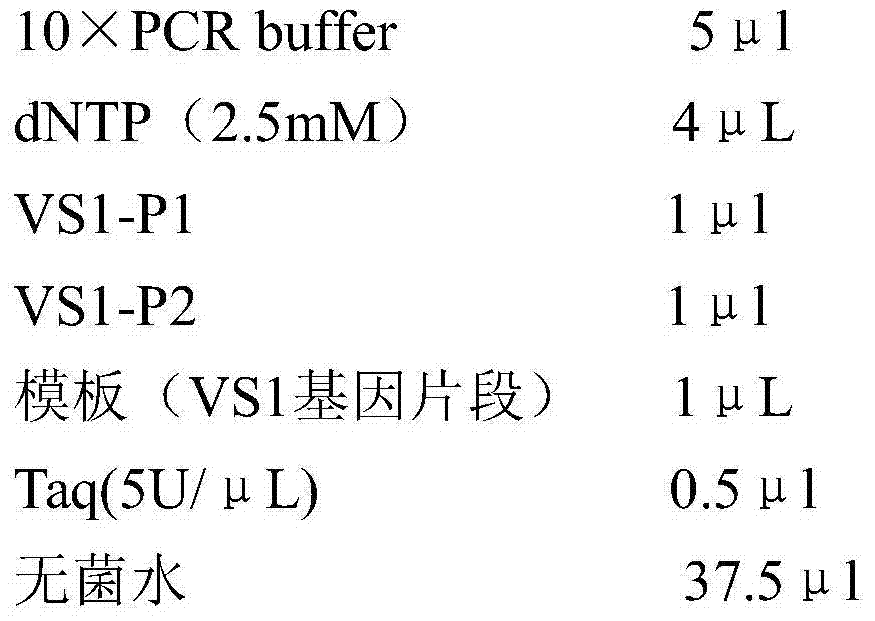
[0017] Example 1 Construction of anti-fish viral hemorrhagic sepsis virus phage single-chain antibody library
[0018] 1. Emulsify the fish viral hemorrhagic septicemia virus obtained by ultracentrifugation with Freund's complete adjuvant 1:1 and immunize BALB / c mice, and boost the immunization twice until the antibody titer reaches 1:100,000 or more. Take the mouse spleen, grind it with liquid nitrogen, add 1ml Trizol reagent to 50-100mg spleen, let stand at room temperature for 5min, add 0.2ml chloroform, shake vigorously repeatedly for 15s, and let stand at room temperature for 2-3min. Centrifuge at 12,000g / min at 4°C for 15min, draw the water phase into another clean 1.5ml centrifuge tube, add 0.5ml of isopropanol and mix by inversion, settle at -20°C for 30-60min, and centrifuge at 12,000g / min for 10min. The precipitate was retained, washed once with 70% ethanol, air-dried, and the precipitated RNA was dissolved in DEPC-treated water.
[0019] 2. Removal of genomic DNA a...
Embodiment 2
[0038] Example 2 Analysis of single-chain antibody diversity
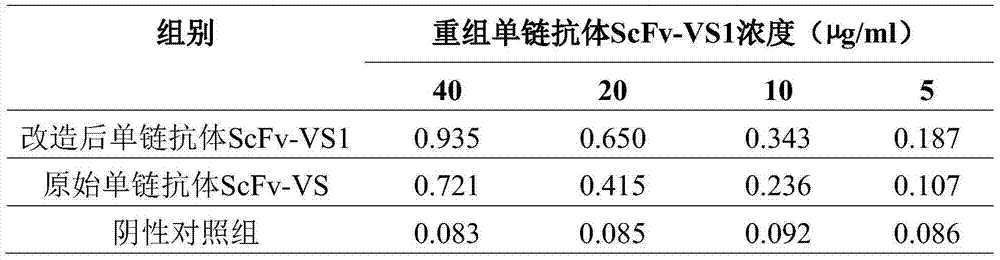
[0039] Ten clones were randomly selected, and the plasmid was extracted after shaking the bacteria, and digested with Sfi I and Not I, and the positive clones were preliminarily identified. Using S1 (S1: 5-CAACGTGAAAAAATTATTATT-3) and S6 (S6: 5-GTAAATGAATTTTCTGTATGAGG-3) as primers for sequencing analysis, the sequencing results of 10 clones showed that the sequences were consistent with the mouse immunoglobulin variable region sequence, Conforms to the mouse light and heavy chain variable region gene structure, the arrangement is VH-Linker-VL. Among them, the VH part is about 357-367bp, the VL is about 320-330bp, and the linker base sequences between the heavy chain and the light chain are all correct. Sequence alignment shows that the homology is more than 80%.
Embodiment 3
[0040] Example 3 Enrichment of Anti-Fish Viral Hemorrhagic Septicemia Virus Single Chain Antibody Library
[0041] 1. The fish viral hemorrhagic septicemia virus cell culture supernatant (titer is 1 × 10 6 PFU / mL) was diluted 1:5 (volume ratio) and coated with carbonate coating buffer to immunotubes, 2ml / tube, overnight at 4°C.
[0042] 2. After coating, wash the tube 3 times with PBS and pat dry; seal the immunotube with blocking (2% skim milk PBS, MPBS), and block at 37°C for 2 hours.
[0043] 3. Pour off the blocking solution, wash the tube 3 times with PBS, and pat dry;
[0044] 4. Mix the supernatant of the primary phage single-chain antibody library obtained above with MPBS and phage supernatant according to the volume ratio of MPBS: supernatant = 2:3, and perform de-interference treatment at room temperature for 20 minutes.
[0045] 5. Add the processed liquid in 4 into the sealed immunotube, 2ml / tube, incubate with gentle shaking for 30min, and then incubate for 1.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com