Purification of recombinant coxsackievirus A16 (CA16) virus-like particles, application thereof in vaccine and vaccine
A virus-like, particle-based technology, applied in the direction of positive-sense single-stranded RNA viruses, viruses, viral peptides, etc., can solve the problems of limiting large-scale production requirements, separation of baculovirus particles, and impact on vaccine quality, achieving strong immunogenicity , good immunogenicity, beneficial to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1 Recombinant CA16 yeast expression strain is fermented and cultivated in a 30L fermenter
[0084] The recombinant Hansenula strain was inoculated into 100 mL primary seed medium (0.67% yeast nitrogen source medium, purchased from SIGMA Company, 0.5% ammonium sulfate, 2% glucose), and cultured at 33° C. on a shaking table at 200 rpm for 20 ~24 hours. Then the whole amount was inoculated in 1000mL secondary seed medium (0.67% yeast nitrogen source medium, 0.5% ammonium sulfate, 2% glycerol), at 33 ° C, 200 rpm shaking culture for 20 to 24 hours (OD 600nm up to 8-10). Then inoculate the whole amount into a 30L fermenter, which contains 12L fermentation medium, adjust the pH value of the fermentation liquid with ammonia water to maintain at 5.0+0.5, the fermentation temperature is 30°C, the rotation speed is controlled at 350-750rpm, and the air flow rate is 0.5-1.0m 3 / h, high-density fermentation requires pure oxygen supplementation, dissolved oxygen is cont...
Embodiment 2
[0091] The separation and purification of embodiment 2CA16 virus-like particles
[0092] Cell collection: the fermentation broth was collected, the precipitate was collected by centrifugation at 6500 rpm, and the cells were washed twice with cell washing buffer.
[0093] Breaking: The collected Hansenula cells were resuspended in cell lysis buffer (20mM NaH 2 PO 4 , 2mM EDTA-Na 2 , 0.2-1.0M NaCl, 2mM PMSF, 0.5% Tween-80, pH6.8-7.4), use a high-pressure homogenizer to break the cells twice under the condition of a pressure of 1200bar, and the cell breakage rate reaches more than 80%.
[0094] Clarification: Pour the crushed cell solution into a centrifuge tube, centrifuge at 7000rpm for 40min, and collect the clarified solution. Or filter the crushed cell fluid through a depth filter at a flow rate of 1300mL / min / m 2 , to collect the clarified solution overnight.
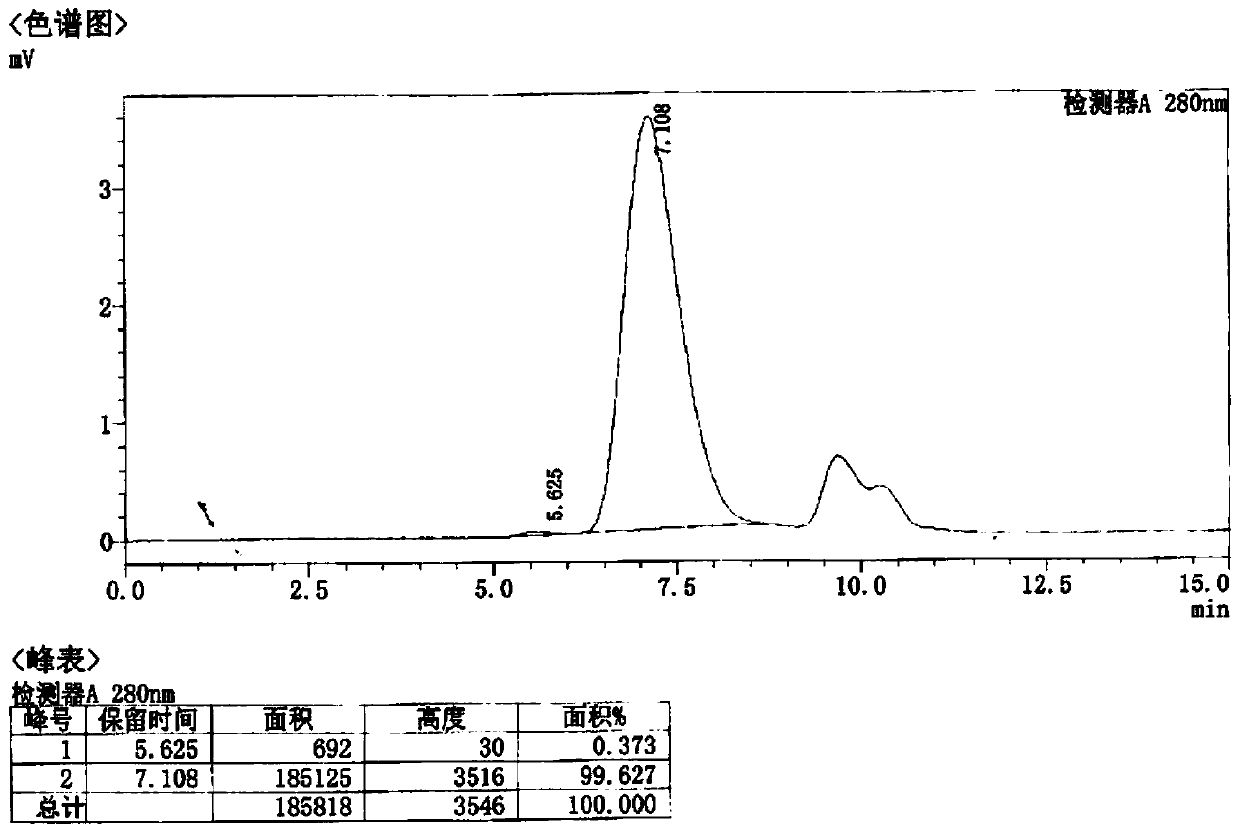
[0095] Ultrafiltration: Use 50mM Tris, 0.25M NaCl and 5% glycerol to form a buffer solution of pH 8.0 to form ...
Embodiment 3
[0109] Preparation of Example 3 Recombinant CA16 VLP Vaccine
[0110] Control of main indicators: Antigen (CA16 virus-like particles) content is 10 μg / mL; aluminum content is controlled at 0.40-0.60 mg / mL; pH value is controlled at 6.6-7.4.
[0111] Preparation method: Dilute the self-made aluminum hydroxide adjuvant to 0.40-0.60 mg / mL with sterile 0.9% sodium chloride solution. Slowly add the purified CA16 stock solution to the adjuvant to make it fully adsorbed, and the vaccine preparation is completed. The prepared vaccine testing standards and testing results are shown in Table 4.
[0112] Table 4 Test results of recombinant CA16 virus-like particle vaccine
[0113] Test items
[0114] The results in Table 4 show that the detection indicators of recombinant CA16 virus-like particle vaccines all meet the detection standards, especially the antigen content is much higher than the minimum standard.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com