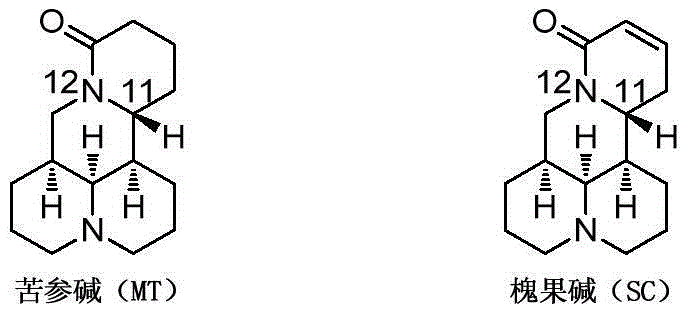Matrine compound derivatives, and preparation method and application thereof
A compound and solvate technology, applied in the field of matrine compound derivatives and their preparation, can solve the problems of large dosage, narrow selective treatment window of sophocarpine, limited action intensity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
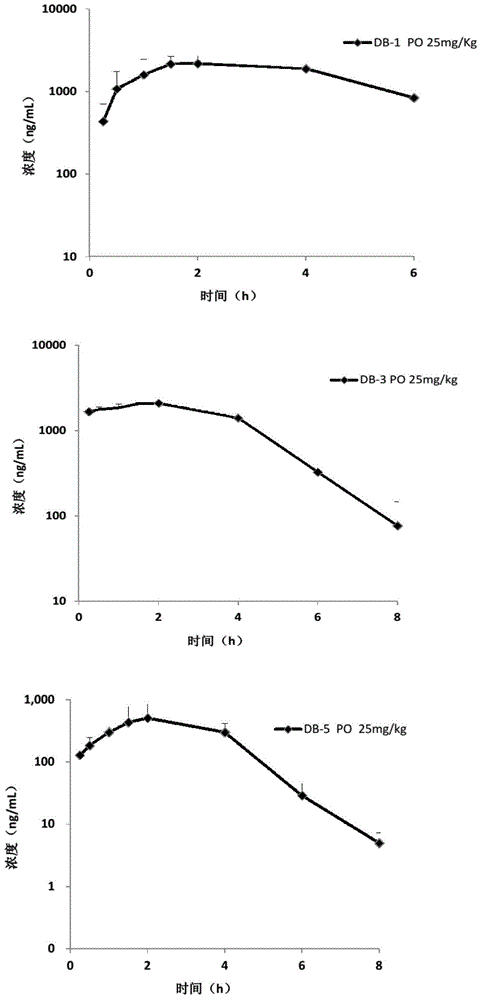
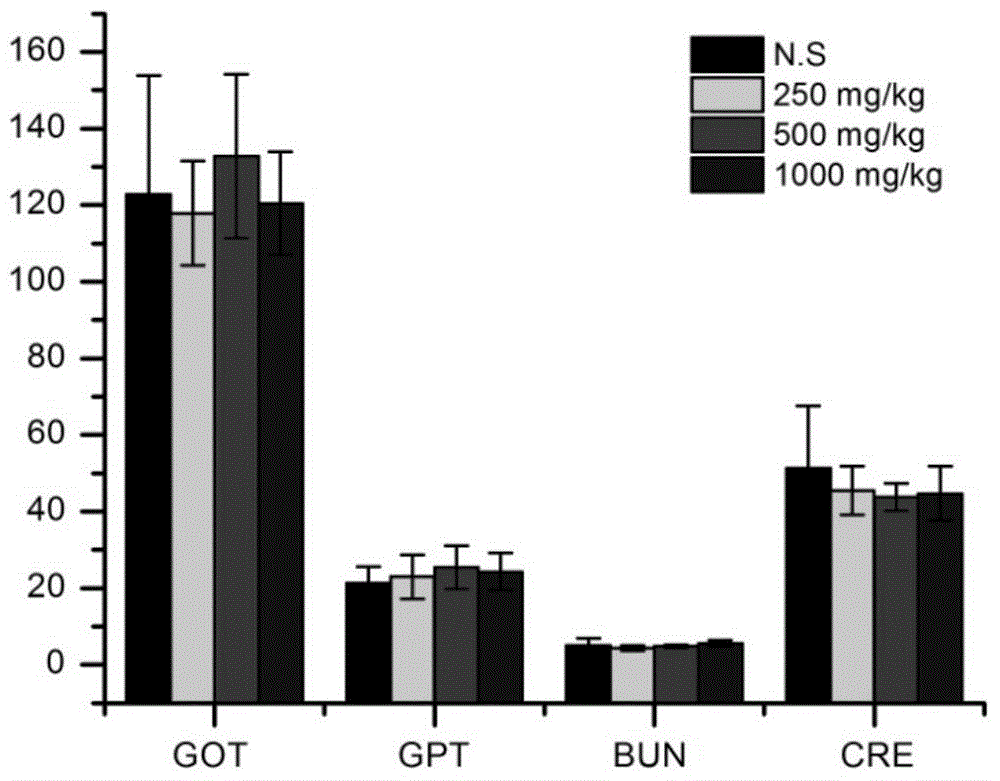
Image
Examples
Embodiment 1
[0171] Example 1 Preparation of 12-N-trifluoromethoxybenzenesulfonyl matrine butyric acid morpholine amide hydrochloride (KCA-1)
[0172]
[0173] Step 1: Preparation of matrine butyric acid (MT-1)
[0174]
[0175] Put matrine (1g, 4mmol) into 20mL 5N NaOH aqueous solution, heat to reflux for 9h, after the reaction, cool to room temperature, a large amount of white solid precipitates, filter, and slowly put the filter cake into 6N HCl, and keep the pH< 5. Evaporate the solvent water to dryness, add methanol to fully dissolve the residue, filter to remove inorganic salts, and obtain a methanol solution of matrine butyric acid, which is directly used in the next reaction.
[0176] Step 2: Preparation of methyl matrine butyrate (MT-2) hydrochloride
[0177]
[0178] Heat the MT-1 methanol solution obtained in step 1 to reflux for 2 hours, and detect it by TLC. After the reaction, evaporate the solvent methanol to dryness, heat 10 mL of absolute ethanol, a large amount ...
Embodiment 2
[0187] Example 2 Preparation of 12-N-trifluoromethoxybenzenesulfonyl matrine butyric acid azetidine amide hydrochloride (KCA-3)
[0188]
[0189] With reference to the operation steps described in Example 1, MT-11-1 and azetidine were reacted under the reaction conditions described in Step 5 and corresponding post-treatments were performed to obtain the target product, a white solid (96.8mg, 45 %); Melting point: 97-99°C. MS-ESI m / z: 530.2; 1 H NMR(400MHz,DMSO)δ10.67(s,1H),7.97(d,J=8.3Hz,2H),7.61(d,J=8.3Hz,2H),4.24-3.86(m,7H),3.91 -3.67(m,2H),3.68-3.53(m,1H),3.17(d,J=19.3Hz,2H),2.88(dd,J=16.5,7.5Hz,2H),2.30-2.20(m,2H ),2.18-2.05(m,1H),2.02-1.80(m,3H),1.77-1.50(m,8H),1.37-1.06(m,2H); 13 C NMR (101MHz, DMSO) δ172.13, 151.39, 140.73, 129.74(2), 121.92(2), 119.03, 63.44, 57.88, 55.06, 54.94, 49.99, 48.98, 47.66, 38.56, 35.27, 30.18, 24.27.62, ,20.87,18.75,18.51,14.98.HRMS: cacld for C26H37N3O5F3S·HCl[M-Cl+H] +:530.2295,found:530.2295.
Embodiment 3
[0190] Example 3 Preparation of 12-N-trifluoromethoxybenzenesulfonyl matrine butyric acid dimethylamine amide hydrochloride (KCA-2)
[0191]
[0192] Referring to the operation steps described in Example 1, MT-11-1 was reacted with dimethylamine under the reaction conditions described in Step 5 and corresponding post-treatment was performed to obtain the target product as a white solid (74 mg, 45%); Melting point: 175-177°C. MS-ESI m / z: 518.2; 1 H NMR(400MHz,DMSO)δ10.84(s,1H),7.97(d,J=8.3Hz,2H),7.55(d,J=8.3Hz,2H),4.23-4.07(m,1H),3.93 -3.78(m,1H),3.77-3.70(m,1H),3.60(d,J=10.1Hz,1H),3.27-3.09(m,2H),3.00-2.90(m,2H),2.83(s ,3H),2.72(s,3H),2.35-2.18(m,3H),2.14-1.99(m,1H),2.00-1.82(m,2H),1.82-1.50(m,8H),1.34-1.10 (m,2H); 13 C NMR (101MHz, DMSO) δ172.01,151.34,140.91,129.76(2),121.73(2),119.01,63.44,58.03,55.07,54.94,49.07,38.54,37.08,35.22,35.10,32.28,247.64,25.64, ,21.34,18.74,18.47.HRMS: calcd for C24H35F3N3O4S·HCl[M-HCl+H] + :518.2295,found:518.2291.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com